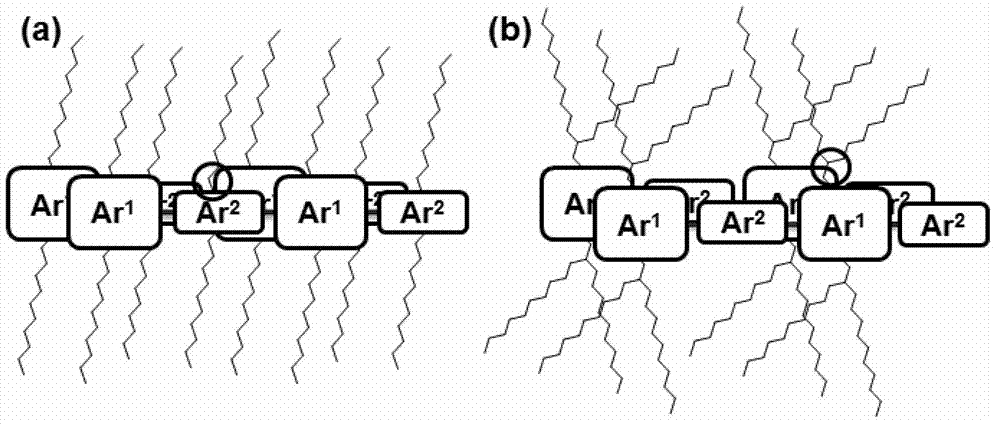
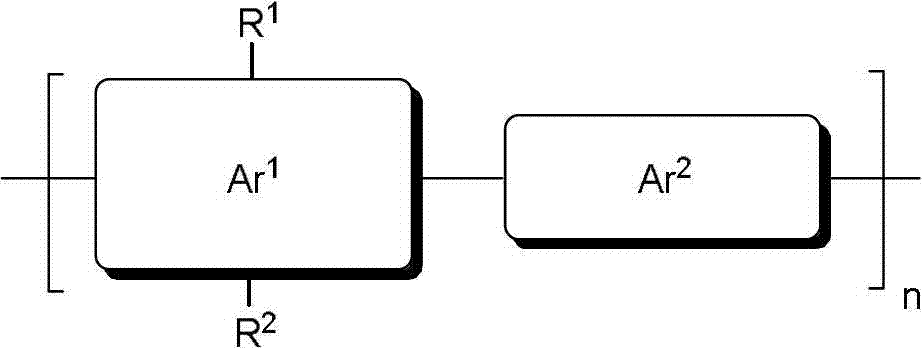
Bifurcate alkyl chain and preparation and application thereof in organic conjugated molecules
A technology of alkyl and alkoxy, which is applied in the preparation of new bifurcated alkyl chains, organic electronic materials and their preparation, and can solve the problem of low polymer mobility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0146]
[0147] The synthetic process of compound 1: add (60g, 0.79mol) 1,3-propanediol in 500ml round-bottomed flask, then add (17.7g, 0.32mol) KOH solid therein, to remove trace moisture in 1,3-propanediol . With stirring at 90°C, add (39.8 g, 0.32 mol) benzyl chloride to 1,3-propanediol using a dropping funnel. Afterwards, the temperature was raised to 130°C for 2 hours, and the reaction was stopped and cooled to room temperature. After the organic phase was extracted by water / ether separation, the solvent was removed by rotary evaporation under reduced pressure, and 39.8 g of a colorless oily product was obtained by distillation under reduced pressure with a yield of 77%. Compound 1. 1 H NMR (CDCl 3 , 400MHz, ppm) δ: 7.36-7.28(m, 5H), 4.51(s, 2H), 3.79-3.75(m, 2H), 3.66-3.64(t, J=5.5Hz, 2H), 2.44(br, s, 1H), 1.88-1.83 (m, 2H).
Embodiment 2
[0149]
[0150] The synthesis process of compound 2: at 0°C, (40g, 0.44mol) 1,4-butanediol was added to 200ml of dry THF, and (5.3g, 0.22mol) sodium hydride was added in batches within 30min, Return to room temperature and react for 2h. Dissolve (38g, 0.22mol) benzyl bromide in 20ml THF, drop it into the above system at 0°C, and then reflux the system for 4h. After the reaction, quench the reaction with cold water, and extract the organic phase with ether. The organic phase was dried with anhydrous sodium sulfate and filtered, and then the solvent was removed by rotary evaporation under reduced pressure, and then 28.1 g of colorless oily liquid 2 was obtained by distillation under reduced pressure with a yield of 71%. 1 H NMR (CDCl 3 , 400MHz, ppm) δ: 7.36-7.26(m, 5H), 4.52(s, 2H), 3.65-3.61(m, 2H), 3.53-3.50(t, J=5.3Hz, 2H), 2.36(br, s, 1H), 1.73-1.65 (m, 4H).
Embodiment 3
[0152]
[0153] The synthesis process of compound 3: at 0°C, (40g, 0.39mol) 1,5-pentanediol was added to 200ml dry THF, and (4.6g, 0.19mol) sodium hydride was added in batches within 30min, Return to room temperature and react for 2h. Dissolve (33g, 0.19mol) benzyl bromide in 20ml THF, drop it into the above system at 0°C, and then reflux the system for 4h. After the reaction, quench the reaction with cold water, and extract the organic phase with ether. The organic phase was dried with anhydrous sodium sulfate and filtered, then the solvent was removed by rotary evaporation, and then 23.1 g of colorless oily liquid 3 was obtained by distillation under reduced pressure with a yield of 62%. 1 HNMR (CDCl 3 , 400MHz, ppm) δ: 7.36-7.26(m, 5H), 4.50(s, 2H), 3.64-3.61(t, J=6.5Hz, 2H), 3.50-3.64(t, J=6.5Hz, 2H) , 1.68-1.54(m, 4H), 1.49-1.43(m, 2H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com