Hepatitis A virus strain HAV-ZL2012, vaccine prepared by using same and application thereof
A technology of hepatitis A virus and virus strains, applied in the field of bioengineering, can solve the problems of long growth cycle and high production cost, and achieve the effects of weakened pathogenicity, shortened replication cycle, and weakened virus virulence
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] The isolation and identification of embodiment 1 hepatitis A virus strain
[0035] The feces of patients in the acute stage of Yanshan County Hospital, Hebei, were dissolved in PBS (pH7.2-7.4), made into a 30-50% suspension, centrifuged, ultracentrifuged with 30% sucrose, and then washed with CsCl 2 Gradient centrifugation, the density gradient is between 1.0 and 1.6g / ml, measure the hemagglutination titer, the hemagglutination titer is 1:32, and further dilute it with 1:5 cell culture medium containing 1.0% fetal bovine serum, and use Filter through a 0.45ml pinhole filter, then sterilize and filter through a 0.22μm pinhole filter, inoculate a monolayer of FRhK6 cells, place the inoculum at 37°C for 4 hours, remove the liquid, and replace it with 0.5% fetal bovine serum, 100IU Qing Damycin, 10 μg streptomycin / ml MEM culture solution, replace the culture solution every week, culture at 37°C for 60 days, use ELISA-TCID 50 Methods The hepatitis A virus antigen was sequen...
Embodiment 2
[0036] Subculture of embodiment 2 hepatitis A virus strain
[0037] 1. After the positive virus strain in Example 1 is passed on the monolayer cells of FRhK6 for 10 times, it is transferred to Vero cells, and the Vero cells are passed to the 10th generation, and the virus culture solution is passed through ELISA-TCID 50 , using the 2B-2C interval of the virus strain genome as a template to design primers for RT-PCR detection, the result was positive, and the virus titer reached 10 4 logELISA-TCID 50 / ml;
[0038] 2. Attenuated adaptation on diploid cells
[0039] The cells obtained in step 1 uploaded to the 10th generation of Vero cells were inoculated with monolayer diploid MRC-5 cells at an MOI of 0.01-0.1, and passed to the 5th-8th generation, and the virus titer could reach 10 7 logELISA-TCID 50 / ml, using the 2B-2C interval of the virus strain genome as a template to design primers for RT-PCR detection, the result is positive, and the diploid cell-adapted strain of th...
Embodiment 3
[0048] Embodiment 3 inactivated vaccine preparation
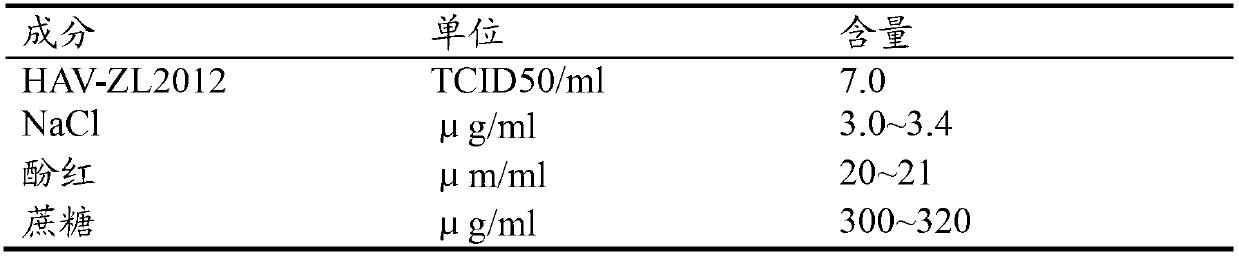
[0049] Using a bioreactor, Vero cells were cultured in suspension with a cell density of 1-2×10 8 ml, the attenuated and adapted hepatitis A virus strain HAV-ZL2012 of the present invention was inoculated at a MOI of 0.01, and the virus was continuously cultured and digested by perfusion for two weeks at 37°C. , 500K medium volume fiber column concentrated SephawsebFF or Eephorose4FF purification or sucrose density continuous flow ultracentrifugation purification, adding formalin solution at 1:4000 (v / v) to inactivate at 37°C for 3 days, residual formalin was washed with double Sodium thiosulfate neutralization, all operations must be carried out under sterile conditions.
[0050] The inactivated vaccine antigen 1m is one dose, and 4 doses are injected into rhesus monkeys intravenously to generate hepatitis A antibody, or 4 doses are injected into rhesus monkeys to challenge the virus.
PUM
| Property | Measurement | Unit |
|---|---|---|
| weight | aaaaa | aaaaa |
| antibody titer | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com