Repaglinide tablet and preparation method thereof
A technology for repaglinide tablets and tablets, applied in the field of pharmaceutical manufacturing, can solve problems such as adverse reactions, and achieve the effects of improving solubility and improving curative effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] prescription:
[0027]
[0028] Preparation method: Mix repaglinide, ethyl oleate, polyoxyethylene hydrogenated castor oil, and polyethylene glycol 4000 evenly, heat to 60°C and stir to dissolve into a solution, add starch, stir evenly, dry at 60°C for 2 hours, and pulverize Pass through an 80 mesh sieve and use it as the intermediate (1) for later use; weigh the microcrystalline cellulose of the prescription amount and mix it with the intermediate (1) evenly, dry-process 20 mesh granulation, 18 mesh granulation, add the prescription amount of cross-linked carboxyl Sodium methylcellulose and magnesium stearate are uniformly mixed and compressed into tablets with a hardness of 5-8kg.
Embodiment 2
[0030] prescription:
[0031]
[0032] Preparation method: Mix repaglinide, ethyl oleate, polyethylene glycol 15 hydroxystearate, meglumine, polyethylene glycol 6000 evenly, heat to 60°C and stir to dissolve into a solution, add calcium hydrogen phosphate, Stir evenly, dry at 60°C for 2 hours, pulverize through an 80-mesh sieve, and use it as an intermediate (1) for later use; weigh the prescribed amount of microcrystalline cellulose and mix it evenly with the intermediate (1), dry-process 20-mesh granulation, 18-mesh Whole grain, add the prescription amount of sodium carboxymethyl starch and magnesium stearate, mix evenly, and compress into a tablet with a hardness of 5-8kg.
Embodiment 3
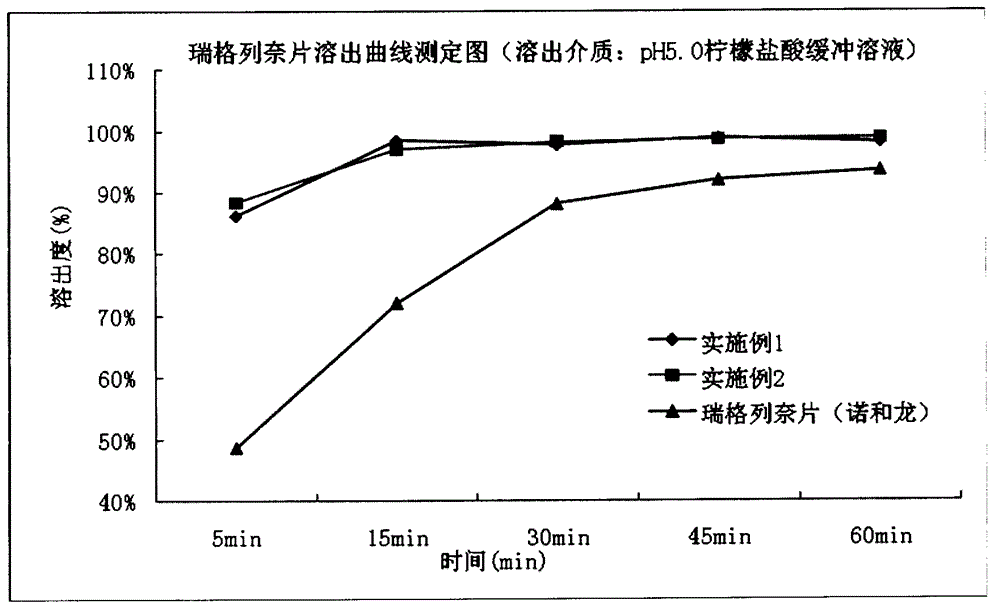
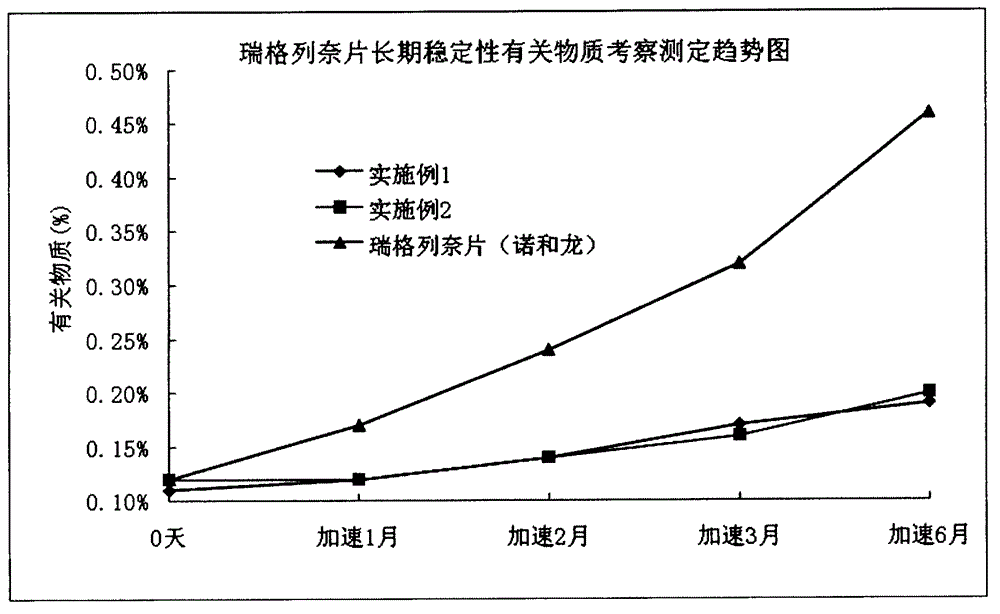
[0033] Example 3: Dissolution testing and long-term stability investigations were performed on the repaglinide tablets prepared in Example 1 and Example 2 and commercially available repaglinide tablets (Novoron).
PUM
| Property | Measurement | Unit |
|---|---|---|
| Hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com