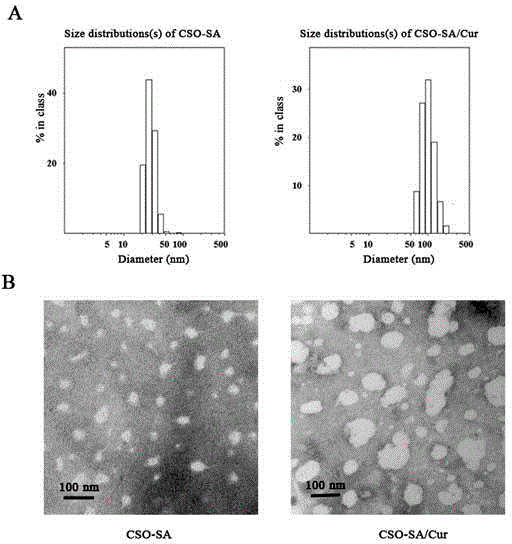
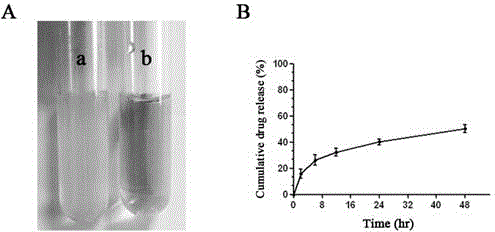
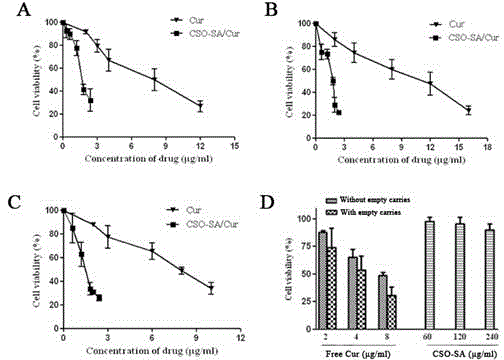
Preparation method and application of curcumin chitosan-stearic acid graft micelle
A technology of chitosan and stearic acid, which is applied in the field of synthesis of polymer prodrugs, can solve the problems of curcumin's low water solubility, low absorption in the body, and low bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0019] The steps of the preparation method of curcumin chitosan stearic acid graft micelles are as follows:
[0020] (1) Synthesis of low molecular weight chitosan
[0021]Get the chitosan 60g that commercially available molecular weight is 450kDa deacetylation degree is 90%, join in the hydrochloric acid aqueous solution of 1.2% in 2000mL concentration by weight, stir 2 hours under the condition of 55 ℃, then add chitosanase 4g, in Perform enzymatic hydrolysis reaction at 55°C, filter with a Buchner funnel, stir at 80°C for half an hour, add 0.5 g of activated carbon, dilute, filter, treat the filtrate with a 0.45 μm microporous membrane, spray dry to obtain chitosan, and measure The weight-average molecular weight that obtains chitosan is 18kDa;
[0022] (2) Synthesis of chitosan-stearic acid graft
[0023] Take 0.8g of chitosan, add 40mL of distilled water and stir to dissolve, add 0.65g of carbodiimide, stir to dissolve, dissolve 0.1g of stearic acid in 20mL of etha...
Embodiment 1
[0029] (1) Synthesis of low molecular weight chitosan (CSO)
[0030] Get the chitosan 60g that commercially available molecular weight is 450kDa deacetylation degree is 90%, join in the hydrochloric acid aqueous solution of 1.2% in 2000mL concentration by weight, stir 2 hours under the condition of 55 ℃, then add chitosanase 4g, in Perform enzymolysis reaction at 55°C, filter with Buchner funnel, stir at 80°C for half an hour, add 0.5g of activated carbon, dilute, filter, treat the filtrate with a 0.45μm microporous membrane, spray dry to obtain chitosan, measure The weight-average molecular weight that obtains chitosan is 18kDa;
[0031] (2) Synthesis of chitosan-stearic acid graft (CSO-SA)
[0032] Take 0.8g of chitosan, add 40mL of distilled water and stir to dissolve, add 0.65g of carbodiimide, stir to dissolve, dissolve 0.1g of stearic acid in 20mL of ethanol solution, mix the above two solutions, and stir magnetically at 400rpm Under certain conditions, react ...
Embodiment 2
[0036] (1) Synthesis of low molecular weight chitosan
[0037] Get commercially available molecular weight is 450kDa deacetylation degree is 90% chitosan 60g, joins in the hydrochloric acid aqueous solution of 1.2% in 2000mL concentration by weight, stirs 2 hours under the condition of 55 ℃, then adds chitosanase 4g, in Perform enzymolysis reaction at 55°C, filter with a Buchner funnel, stir at 80°C for half an hour, add 0.5g of activated carbon, dilute, filter, process the filtrate with a 0.45μm microporous membrane, spray dry to obtain chitosan, and measure The weight-average molecular weight that obtains chitosan is 18kDa;
[0038] (2) Synthesis of chitosan-stearic acid graft
[0039] Take 0.8g of chitosan, add 40mL of distilled water and stir to dissolve, add 0.65g of carbodiimide, stir to dissolve, dissolve 0.1g of stearic acid in 20 mL of ethanol solution, mix the above two solutions, and magnetically Under stirring conditions, react at a constant temperature ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| The average particle size | aaaaa | aaaaa |
| Weight average molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com