Recombinant hirudin eye drops and preparation method thereof
A technology for reconstituting hirudin and eye drops, which is applied in the directions of drug combinations, pharmaceutical formulations, medical preparations containing active ingredients, etc. Clarity and storage stability, preventing liquid spillage and loss, moisturizing and comfortable ocular surface
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Formula of recombinant hirudin eye drops:
[0022]
[0023] Preparation Process:
[0024] 1. Under the condition of 60-80℃ water bath, stir quickly and slowly add 0.1g of sodium hyaluronate into 70ml of water for injection to make it completely dispersed and fully swell, so as not to make it stick together, stir for about 30 minutes can be completely dissolved.
[0025] 2 Add boric acid, borax, sodium chloride, sodium edetate and ethyl p-hydroxybenzoate to the above sodium hyaluronate solution, stir well to dissolve, and then set the volume to 100ml to obtain pH7.4 Eye drops blank matrix.
[0026] 3. Add the recombinant hirudin III to the blank matrix of the above-mentioned eye drops, stir well to dissolve, filter and sterilize through a 0.22um filter, and then subpackage to obtain the recombinant hirudin III eye drops.
Embodiment 2
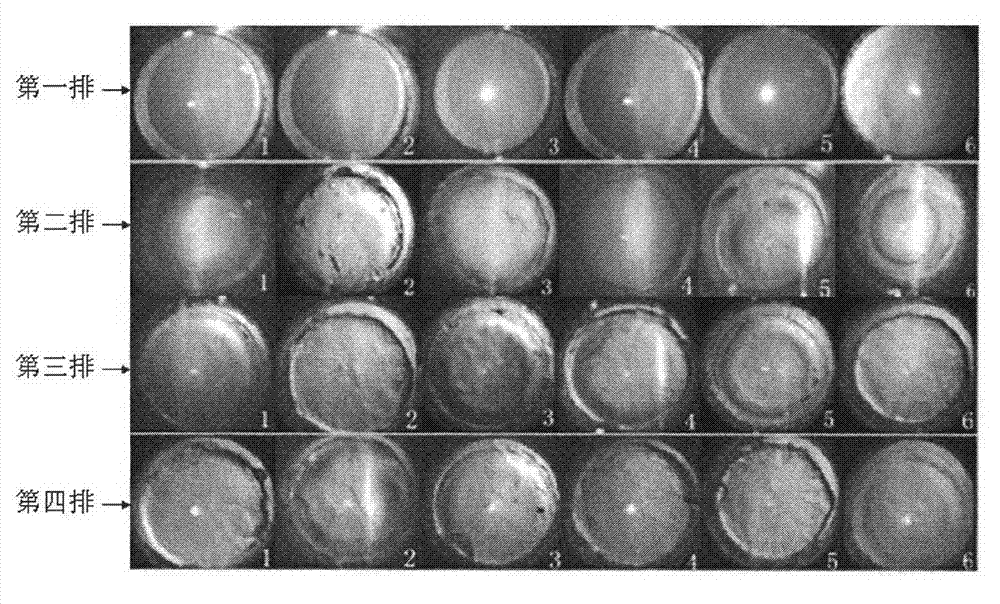
[0028] Rabbit Eye Irritation Test
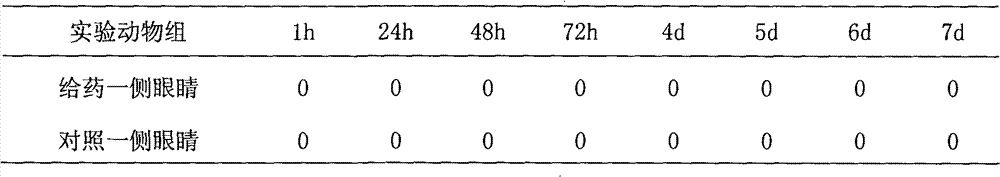
[0029] Recombinant hirudin III eye drops in embodiment 1 according to the new drug preclinical guideline method [4] For the irritation test, select 4 healthy New Zealand rabbits, give recombinant hirudin III eye drops 5 times a day to the left eye, and give normal saline to the right eye as a blank control, and perform eye examinations at 1, 24, 48, and 72 hours to observe The conjunctiva, cornea, iris and other visible injuries were continuously observed for 7 days, and the eye reaction scores were recorded according to the eye irritation reaction score standard (results are shown in Table 1).
[0030] Table 1 The final scores of the eyes of each group at each observation time
[0031]
[0032] Conclusion: The cornea, iris and conjunctiva of the eye receiving the recombinant hirudin III eye drops have no irritation, which is the same as the eye of the control side, thus proving that the recombinant hirudin III eye drop has no eye irrita...
Embodiment 3
[0034] Stability test
[0035] After the anticoagulant activity of the recombinant hirudin III eye drops was measured, they were placed in a refrigerator at 4°C and at room temperature. After 1, 2, 4 weeks and 3, 6, and 12 months, the anticoagulant activity and pH value did not change. Appearance shape, clarity and sterility test all meet the requirements of appendix IIIA of Pharmacopoeia of the People's Republic of China (Part Two) for eye drops.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com