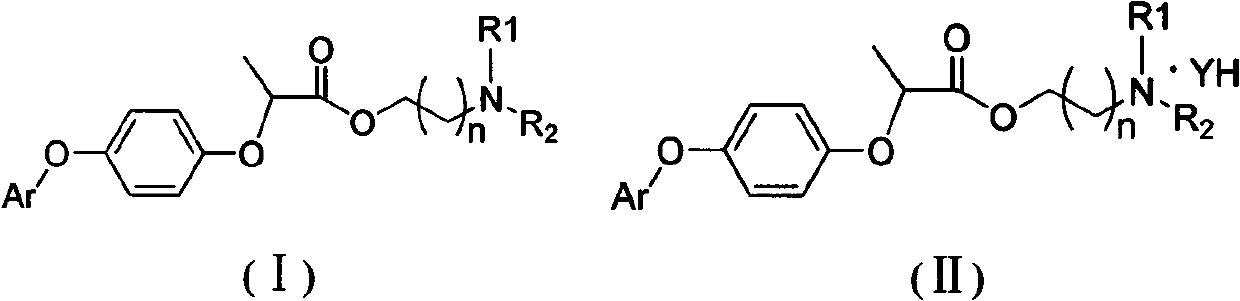
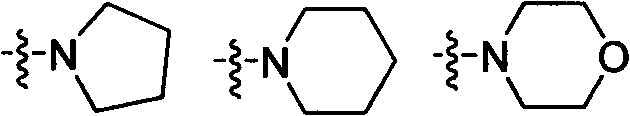
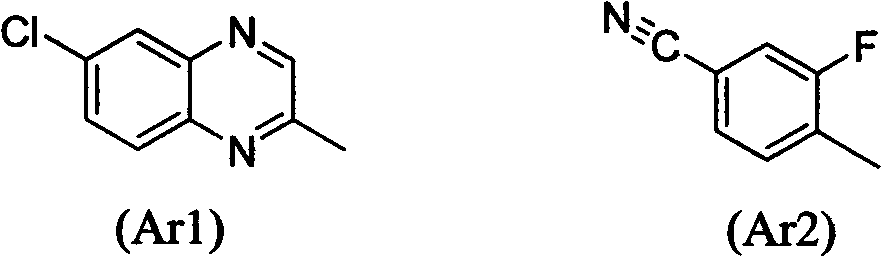Preparation and application research of water-soluble and oil-soluble novel aryloxy phenoxy carboxylate derivatives
An aryloxyphenoxycarboxylic acid and ester technology is applied to the preparation and herbicidal activity of novel aryloxyphenoxycarboxylic acid ester derivatives. It can solve the problems of difficult preparation processing, and achieve the effect of controlling growth, good herbicidal activity and broad-spectrum.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Synthetic example
[0054] Synthesis of compound 1:
[0055] 1.
[0056]
[0057] Add III (R type, 18.64g, 0.05mol, commercially available) and 40ml THF into a 250ml reaction flask, stir until completely dissolved, add dropwise 2.2g of sodium hydroxide in 40ml of aqueous solution, stir at room temperature for 4 hours, stop stirring, and filter with suction Insoluble matter was filtered off, THF was removed under reduced pressure, and the aqueous solution was acidified with dilute hydrochloric acid to obtain 16.3 g of white solid (III-1).
[0058] 2.
[0059]
[0060] Add III-1 (1.72g, 0.005mol) and 15ml of dichloromethane into a 50ml reaction flask, add thionyl chloride (1.18g, 0.01mol) and 2 drops of DMF under stirring, heat and reflux for 5 hours, and the solvent is yellow Oil (III-2) 1.70g.
[0061] 3.
[0062]
[0063] Add III-2 (1.70g, 0.0047mol) into a 50ml reaction flask, add 10ml of dichloromethane and stir to dissolve, add dropwise morpholine propanol (0.70g), triethylamine ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com