A kind of aryloxyphenoxypropionate compound and its preparation method and application
A technology of aryloxyphenoxypropionate and aryloxyphenoxy, which is applied in the field of aryloxyphenoxypropionate compounds and their preparation and application, and can solve the problems of no research and development reports on herbicidal activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Preparation of pepper rings
[0036] Under the catalysis of tetrabutylammonium bromide (TBAB), dimethyl sulfoxide is used as a solvent, mixed with dichloromethane evenly, and the reaction is heated to 110 ° C, and dimethyl sulfoxide containing catechol is added dropwise to it at the same time. The sulfone solution and 50% inorganic alkali solution continue to react for about 3 hours, then stop the reaction, add water, continue heating and distilling, and collect fractions at 99-104°C. The distillate was treated to obtain pepper rings with a yield of 85.6%.
[0037] Preparation of piperonal:
[0038] Synthesis of N-methylbenzamide from N-methylaniline: Put 5.0g N-methylaniline, 5.0g 85% formic acid and 20ml toluene into a 100ml three-necked flask, and slowly raise the temperature to reflux under stirring. After about 5 minutes of reflux, the reflux liquid is continuously separated from the water through the water separator, and the toluene is returned to the reaction b...
Embodiment 2
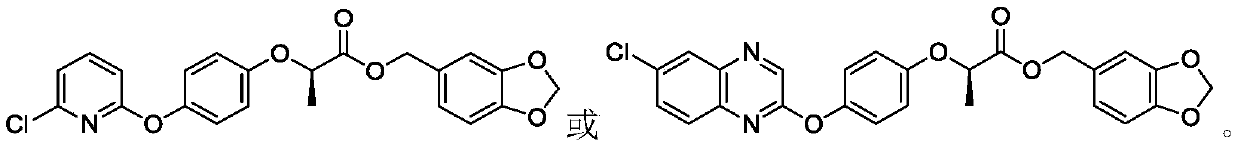
[0043] Preparation of (R)-benzo[d][1,3]dioxa-5-methyl-2-(4-(6-chloroquinoxalin-2-yloxy)phenoxy)propionate
[0044]
[0045]0.152g (1.0mmol) of 3,4-methylenedioxybenzyl alcohol was dissolved in 30mL of dichloromethane, after stirring for 5min, 1.0mmol of triethylamine was added, and a catalytic amount of 4-dimethylaminopyridine (DMAP) was added in an ice bath Add dropwise a 10mL dichloromethane solution containing 1.2mmol 2-(4-(6-chloroquinoxalin-2-yloxy)phenoxy)propionyl chloride under the conditions, and drop it in about 25 minutes. After 3.0 hours of reaction, the reaction is complete. The organic layer was washed once with dilute hydrochloric acid, washed three times with saturated brine, and the organic layer was washed with Na 2 SO 4 dry. Desolvation, the crude product was subjected to column chromatography [V 石油醚 :V 乙酸乙酯 =7:1~5:1] to obtain white solid (R)-benzo[d][1,3]diox-5-methyl-2-(4-(6-chloroquinoxalin-2-yloxy Base) phenoxy) propionate, m.p.111~113°C, yield ...
Embodiment 3
[0047] Preparation of (R)-benzo[d][1,3]dioxa-5-methyl-2-(4-((6-chloropyridin-2-yl)oxy)phenoxy)propionate
[0048]
[0049] The preparation method is the same as in Example 2, 0.152g (1.0mmol) 3,4-methylenedioxybenzyl alcohol, 1.0mmol triethylamine, catalytic amount of 4-dimethylaminopyridine (DMAP), 20mL dichloromethane, Add 1.2mmol (R)-2-(4-((6-chloropyridin-2-yl)oxy)phenoxy)propionyl chloride solution in dichloromethane dropwise under ice-bath condition, drop it in about 15 minutes, and Reaction 1.0h. After completion of the reaction, the organic layer was washed with dilute hydrochloric acid, washed with saturated brine, dried, and precipitated, and the crude product was subjected to column chromatography [V 石油醚 :V 乙酸乙酯 =7:1~5:1] to obtain oily liquid (R)-benzo[d][1,3]diox-5-methyl-2-(4-((6-chloropyridin-2-yl) Oxygen) phenoxy) propionate, yield 75.1%. 1 H NMR (400MHz, CDCl 3 )δ: 7.59 (d, J=2.2Hz, 1H, pyridine-H),) 6.97-7.08 (m, 3H, C 6 h 4 , pyridine-H), 6.86 (d...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com