Cyclohexadiene oxime ether compound and application thereof
A technology of cyclohexadiene oxime ether and compound, applied in the field of pharmaceutical synthesis, can solve the problems of limited application scope, unusable herbicides and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
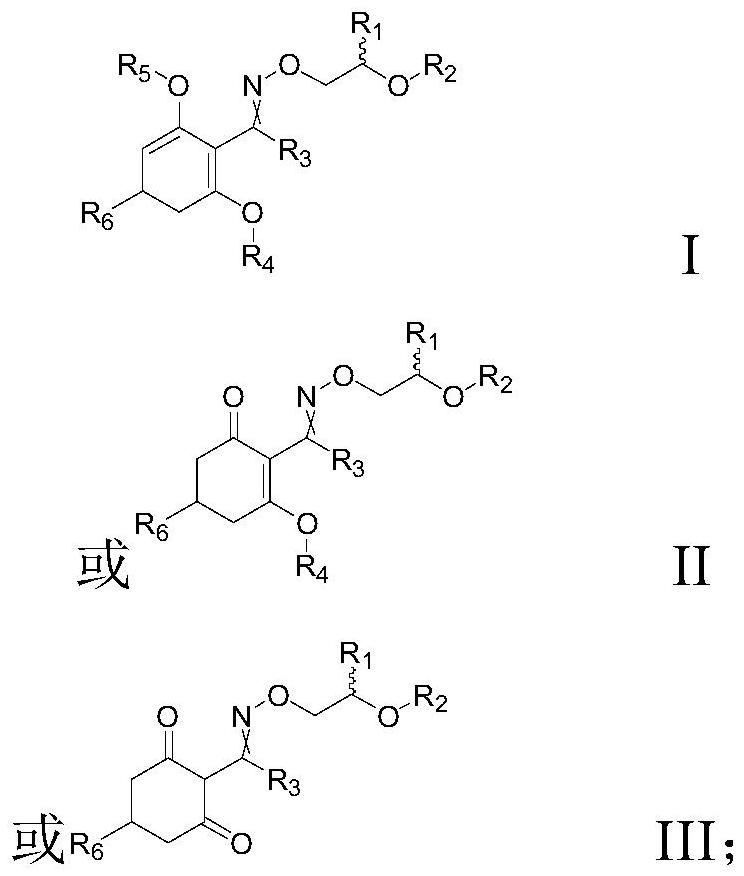
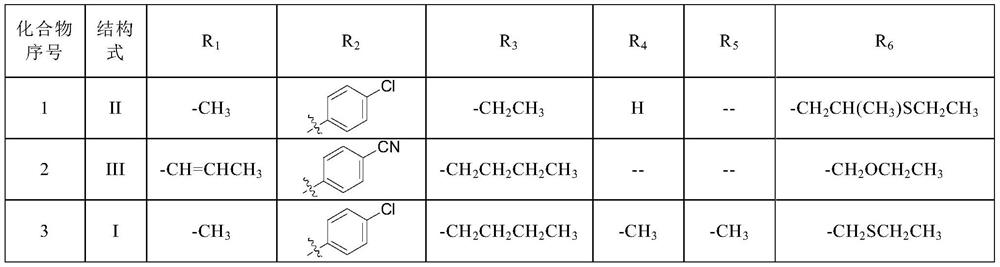
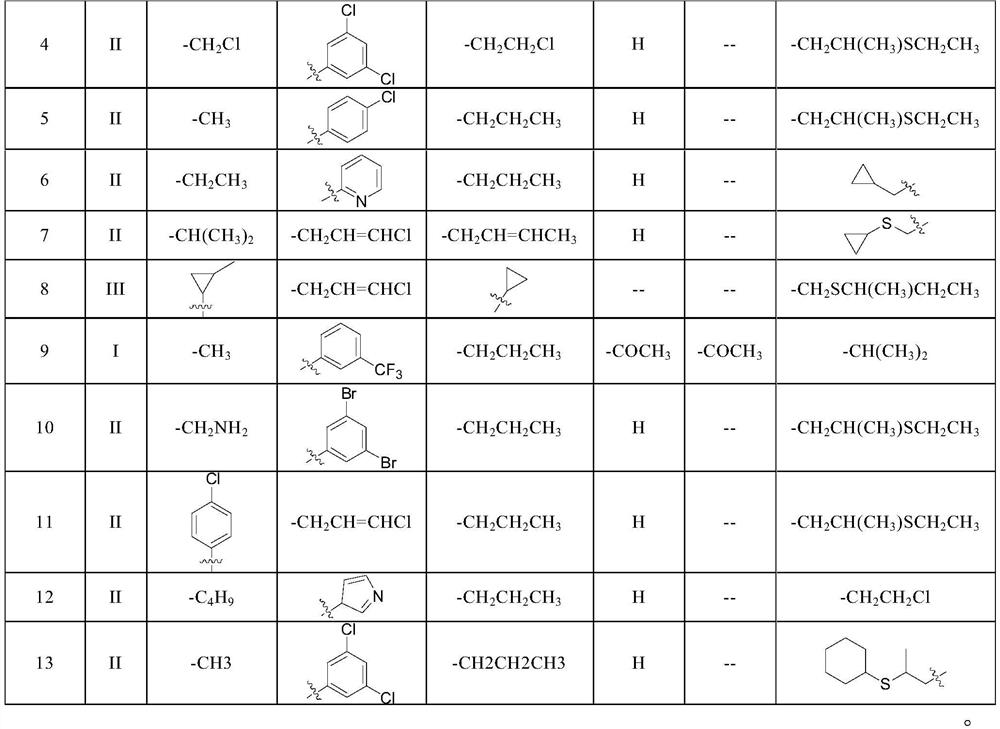
[0041] This embodiment provides a kind of compound 1, and the structural formula of this compound 1 is as follows:
[0042]
[0043] The synthetic method of above-mentioned compound 1 comprises: in reactor, add 14.8g O-[2-(4-chloro-phenoxy group)-propyl group]-hydroxylamine and 19g propionyl triketone, 100ml sherwood oil, 5ml acetic acid, in The reaction was carried out at 25° C. for 24 hours. After the reaction, the solvent was removed, washed with water and concentrated to obtain 31 g of a reddish-brown viscous liquid, namely compound 1, with a yield of 97%.
[0044] Wherein, the proton nuclear magnetic spectrum of the compound 1: 1HNMR (400MHz CDCl3): δ7.2 (2H, m), δ6.8 (2H, m), δ4.6 (1H, m), δ4.1 (2H, m),δ2.8(2H,m),δ2.5(2H,m),δ2.2(2H,m),δ2.1(1H,m),δ1.5(2H,m),δ1. 2(11H,m), δ0.9~1.1(4H,m).
Embodiment 2
[0046] This embodiment provides a kind of compound 2, and the structural formula of this compound 2 is as follows:
[0047]
[0048] The synthetic method of above-mentioned compound 2 comprises: in reactor, add 15.3g 4-(1-aminooxymethyl-butyl-2-enoxy)-benzonitrile and 17.8g 5-ethoxymethyl-2 -Verylcyclohexane-1,3-dione, 150ml petroleum ether, 3ml acetic acid, reacted at 30°C for 12h, removed the solvent after the reaction, washed with water and concentrated to obtain 30g of reddish-brown viscous liquid, namely compound 2, and The yield was 94%.
[0049] Wherein, the proton nuclear magnetic spectrum of the compound 2: 1HNMR (400MHz CDCl3): δ7.3 (2H, d), δ6.9 (2H, d), δ5.5 (2H, m), δ4.1 (1H, m), δ3.8(2H,d), δ3.3(5H,m), δ2.3(5H,m), δ1.8(3H,d), δ1~1.3(12H,m).
Embodiment 3
[0051] This embodiment provides a kind of compound 3, and the structural formula of this compound 3 is as follows:
[0052]
[0053] The synthetic method of above-mentioned compound 3 comprises: in reactor, add 14g O-[2-(4-chloro-phenoxy)-propyl]-hydroxylamine and 20.9g 1-(4-ethylthiomethyl-2, 6-dimethoxy-cyclohexyl-1,5-dienyl)-pentan-1-one, 120ml petroleum ether, 15ml acetic acid, react at 15°C for 28h, remove the solvent after the reaction, wash with water and concentrate to get 31 g of reddish-brown viscous liquid, namely compound 3, and the yield was 92%.
[0054] Wherein, the proton nuclear magnetic spectrum of the compound 3: 1HNMR (400MHz CDCl ): δ7.2 (2H, d), δ6.6 (2H, d), δ4.6 (1H, d), δ3.8 (3H, m), δ3.4 (6H, s), δ2.2 (7H, m), δ1.5 (3H, d), δ1.2~1.4 (9H, m), δ0.9 (3H, t).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com