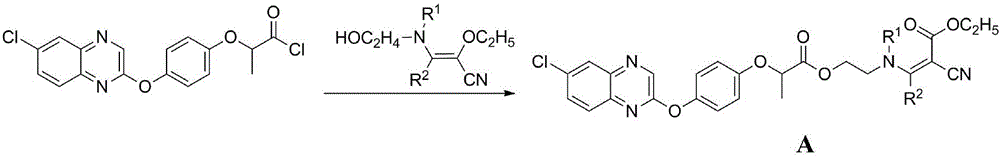
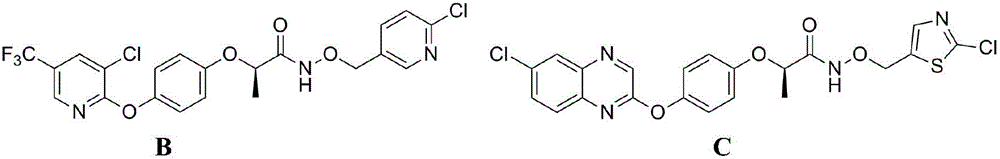
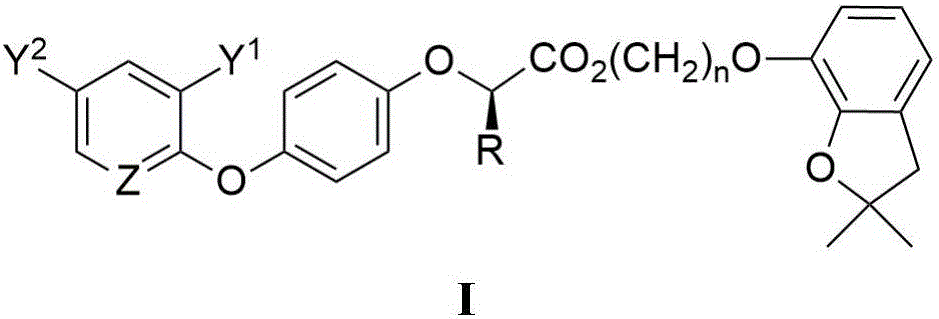
2-aryloxy carboxylic acid(dihydrobenzofuran-7-phenoxyl)alkyl ester
A technology of aryloxycarboxylic acid and dihydrobenzene, which is applied in biocides, plant growth regulators, animal husbandry and other directions, can solve the problem that 2-aryloxycarboxylic acid herbicidal activity is not reported and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Preparation of 2-(2,2-dimethyl-2,3-dihydrobenzofuran-7-oxyl)ethanol
[0027]
[0028] 10mmol furanol, 40mmol 2-chloroethanol, 20mmol K 2 CO 3 React with 0.5mmol PEG-200, 20mL ethanol, 70°C for 10.0h. TLC detection, the reaction is complete, cooling, filtering to remove K 2 CO 3 , the filtrate was desolvated, the residue was dissolved in 40mL of dichloromethane, the organic layer was washed with water and dried, and the desolvated dark green solid 2-(2,2-dimethyl-2,3-dihydrobenzofuran-7-oxo Base) ethanol, m.p.42~45 ℃, yield 93.2%; 1 H NMR (400MHz, CDCl 3 )δ: 1.41(s, 6H, 2×CH 3 ), 2.93 (s, 2H, CH 2 ), 3.80~3.83 (m, 2H, OCH 2 ), 4.03~4.05 (m, 2H, CH 2 ), 6.66~6.71 (m, 3H, C 6 h 3 ).
Embodiment 2
[0030] Preparation of 3-(2,2-Dimethyl-2,3-dihydrobenzofuran-7-oxyl)propanol
[0031]
[0032] Operation is with embodiment 2, 0.84g (5.1mmol) furan phenol and 2.84g (20.4mmol) 3-bromopropanols, 1.41g (10.2mmol) K 2 CO 3 , and reacted for 7.0h to obtain 0.94g of light green solid 3-(2,2-dimethyl-2,3-dihydrobenzofuran-7-oxyl)propanol 6b, m.p.52~55℃, yield 83.2 %; 1 H NMR (400MHz, CDCl 3 )δ: 1.49(s, 6H, 2×CH 3 ), 2.00~2.07 (m, 2H, CH 2 ), 3.02(s, 2H, CH 2 ), 3.84~3.88 (m, 2H, OCH2 ), 4.18~4.22 (m, 2H, CH 2 ), 6.75~6.80 (m, 3H, C 6 h 3 ).
Embodiment 3
[0034] Preparation of 4-(2,2-Dimethyl-2,3-dihydrobenzofuran-7-oxyl)butanol
[0035]
[0036] 0.164g (1.0mmol) furanol and 0.43g (4.0mmol) 4-chlorobutanol, 0.276g (2.0mmol) K 2 CO 3 , catalytic amount of TBAB, 20mL acetone, reflux for 12.0h, cooling, suction filtration, drying, precipitation, crude product through column chromatography [V 石油醚 :V 乙酸乙酯 =5:1] Column chromatography yielded 0.18 g of white solid 4-(2,2-dimethyl-2,3-dihydrobenzofuran-7-oxyl)butanol, m.p.62-65°C, yield 76.2%; 1 H NMR (400MHz, CDCl 3 )δ: 1.50(s, 6H, 2×CH 3 ), 1.73~1.78 (m, 2H, CH 2 ), 1.86~1.93 (m, 2H, CH 2 ), 3.01 (s, 2H, CH 2 ), 3.71(t, J=6.4Hz, 2H, OCH 2 ), 4.09(t, J=6.4Hz, 2H, CH 2 ), 6.73~6.76 (m, 3H, C 6 h 3 ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com