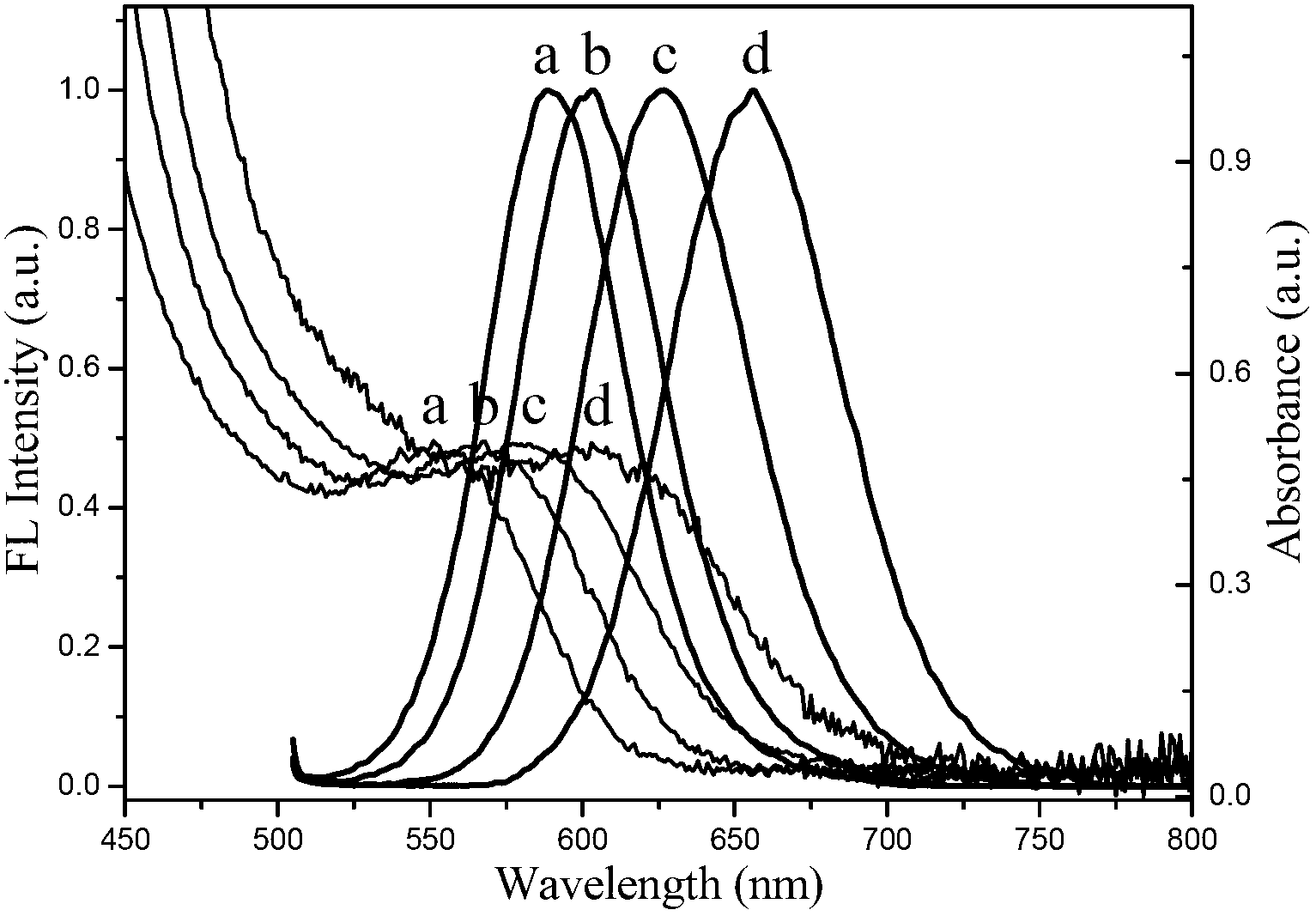
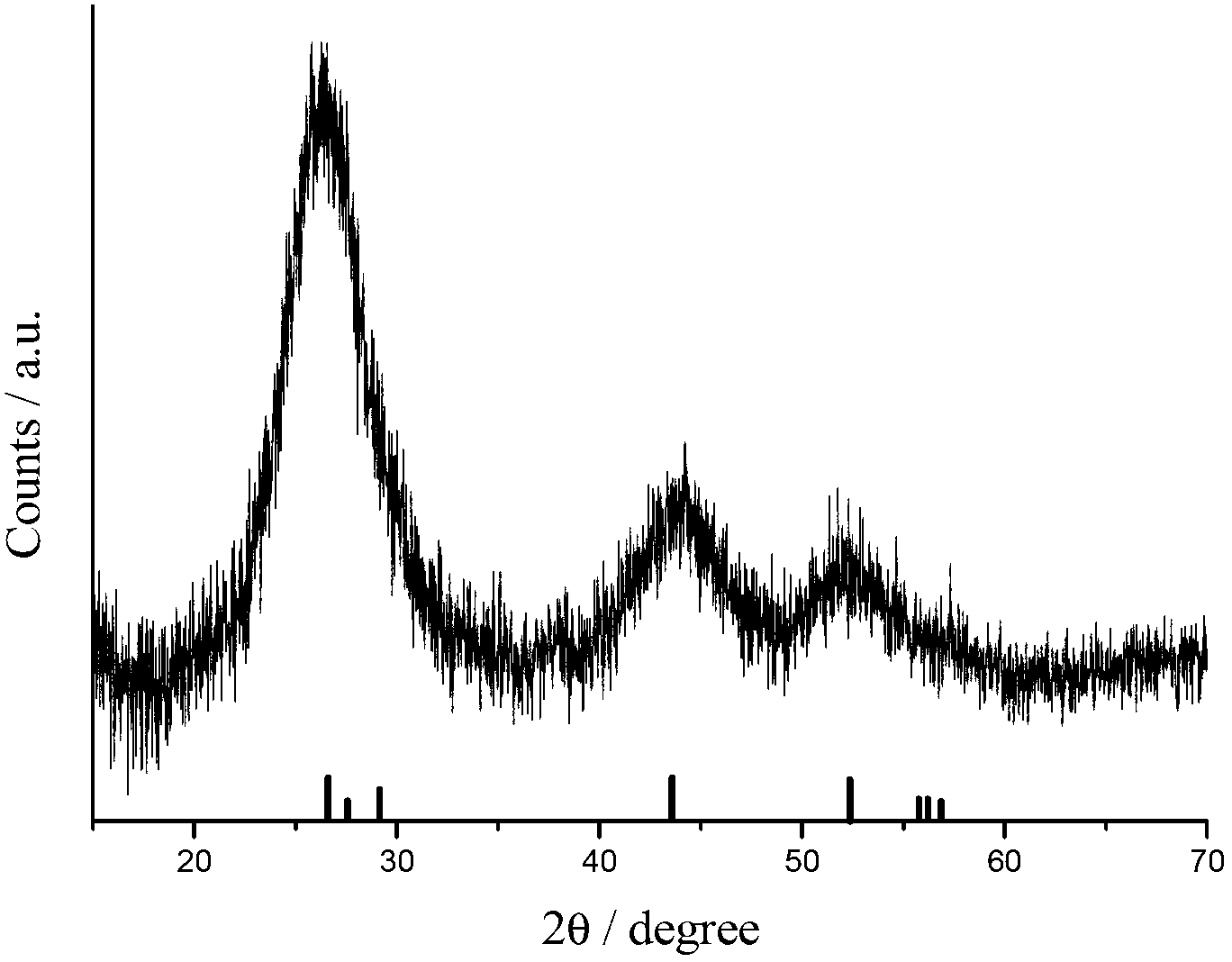
Preparation method of water soluble CdTe/CdS/ZnS nuclear/shell/shell type quantum dot
A water-soluble, quantum dot solution technology, applied in the field of nanomaterials, can solve the problems of poor fluorescence performance, poor biocompatibility, volatile odor, etc., and achieve high fluorescence quantum yield, good biocompatibility, and simple operation Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] A preparation method of water-soluble CdTe / CdS / ZnS core / shell / shell type quantum dots, the steps are as follows:
[0026] (a) Prepare sodium tellurium hydride NaHTe solution as tellurium source: sodium borohydride NaBH with a molar ratio of 2:1 4 Put the tellurium powder Te in water, under the protection of nitrogen, stir and react at 80°C for 1h to obtain a sodium telluride hydride NaHTe solution;
[0027] (b) Under the protection of nitrogen, sodium hydride telluride NaHTe solution was added to cadmium chloride and N-acetyl-L-cysteine solution at pH 12.0, so that Cd 2+ : N-acetyl-L-cysteine: the mol ratio of NaHTe is 1: 2: 0.05, at 90 ℃ of reflux 10min, solution is cooled to room temperature, obtains CdTe quantum dot solution;
[0028] (c) Under the protection of nitrogen, add N-acetyl-L-cysteine, cadmium chloride and sodium sulfide to the CdTe quantum dot solution obtained in the second step, and adjust the pH value to 12.0, so that Cd 2+ : N-acetyl-L-cysteine: S...
Embodiment 2
[0031] A preparation method of water-soluble CdTe / CdS / ZnS core / shell / shell type quantum dots, the steps are as follows:
[0032] (a) Prepare sodium tellurium hydride NaHTe solution as tellurium source: sodium borohydride NaBH with a molar ratio of 2:1 4 Put the tellurium powder Te in water, under the protection of nitrogen, stir and react at 80°C for 1h to obtain a sodium telluride hydride NaHTe solution;
[0033] (b) Under the protection of nitrogen, sodium hydride telluride NaHTe solution was added to cadmium chloride and N-acetyl-L-cysteine solution at pH 12.0, so that Cd 2+ : N-acetyl-L-cysteine: the mol ratio of NaHTe is 1: 2: 0.05, at 90 ℃ of reflux 10min, solution is cooled to room temperature, obtains CdTe quantum dot solution;
[0034] (c) Under the protection of nitrogen, add N-acetyl-L-cysteine, cadmium chloride and sodium sulfide to the CdTe quantum dot solution obtained in the second step, and adjust the pH value to 12.0, so that Cd 2+ : N-acetyl-L-cysteine: S...
Embodiment 3
[0037] A preparation method of water-soluble CdTe / CdS / ZnS core / shell / shell type quantum dots, the steps are as follows:
[0038] (a) Prepare sodium tellurium hydride NaHTe solution as tellurium source: sodium borohydride NaBH with a molar ratio of 2:1 4Put the tellurium powder Te in water, under the protection of nitrogen, stir and react at 80°C for 1h to obtain a sodium telluride hydride NaHTe solution;
[0039] (b) Under the protection of nitrogen, sodium hydride telluride NaHTe solution was added to cadmium chloride and N-acetyl-L-cysteine solution at pH 12.0, so that Cd 2+ : N-acetyl-L-cysteine: the mol ratio of NaHTe is 1: 2: 0.05, at 90 ℃ of reflux 10min, solution is cooled to room temperature, obtains CdTe quantum dot solution;
[0040] (c) Under the protection of nitrogen, add N-acetyl-L-cysteine, cadmium chloride and sodium sulfide to the CdTe quantum dot solution obtained in the second step, and adjust the pH value to 12.0, so that Cd 2+ : N-acetyl-L-cysteine: S ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com