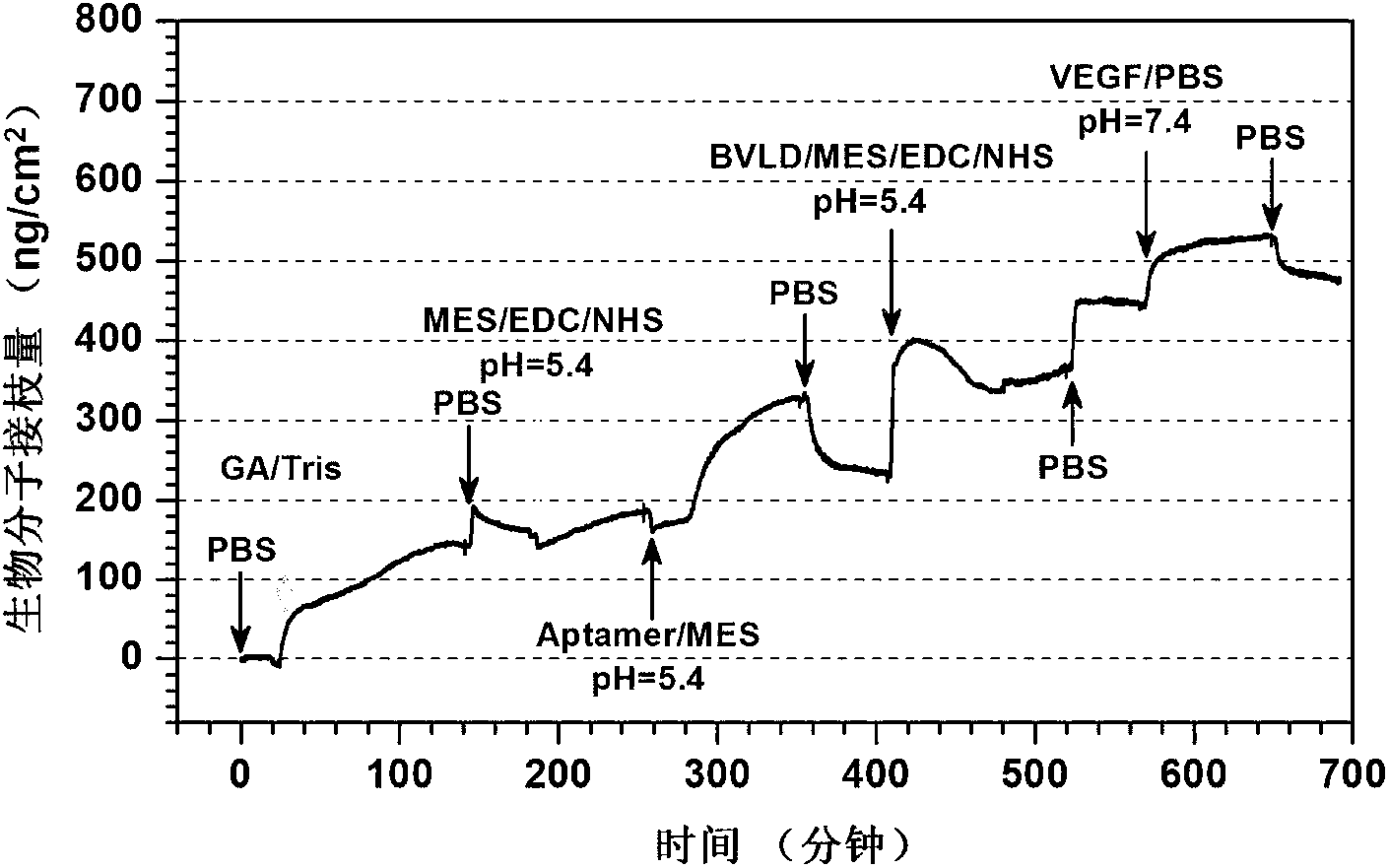
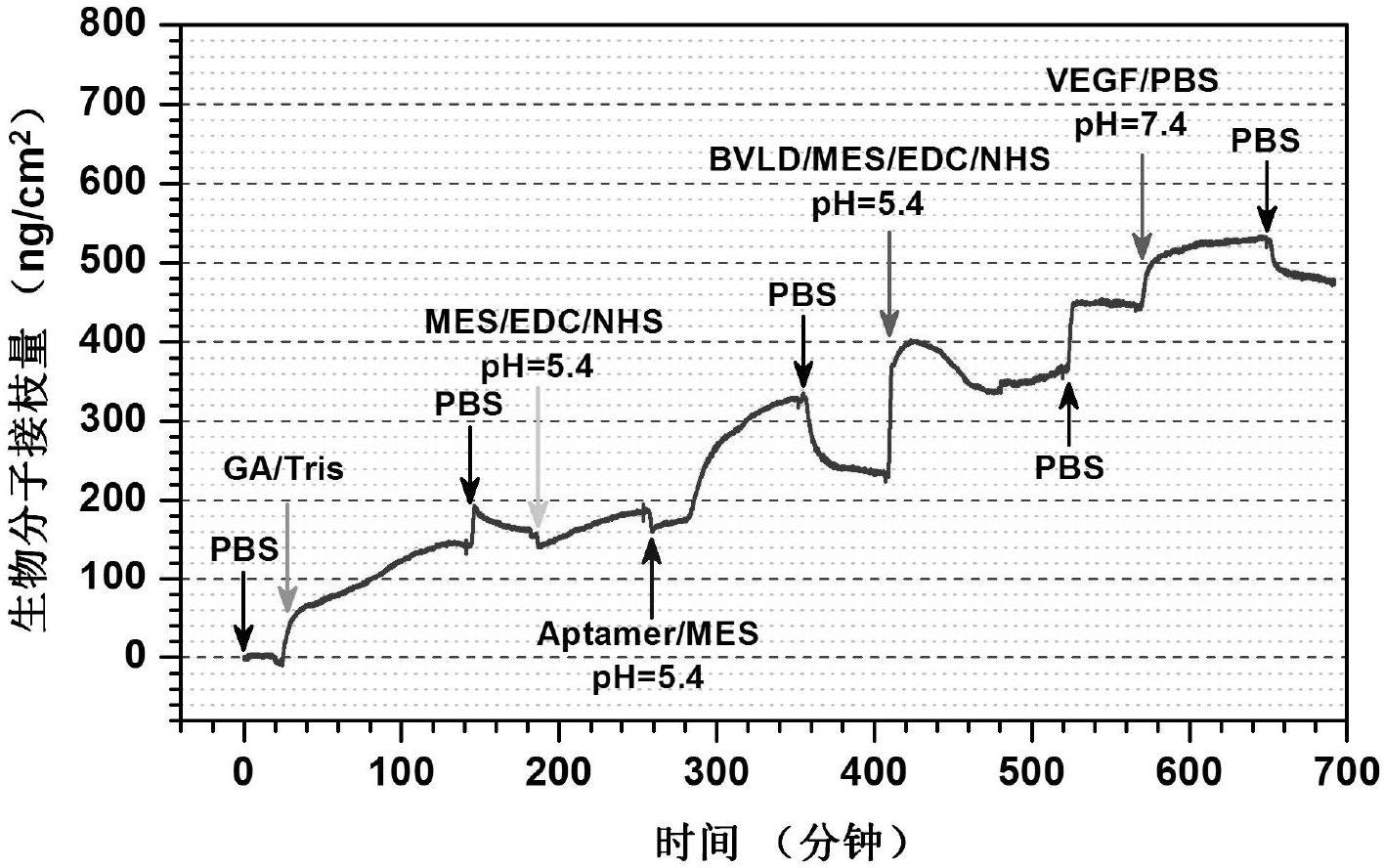
Preparation method of biomedical materials of multiclass functional group rich in amino group, carboxyl group and benzoquinonyl group
A biomedical material and amino-rich technology, which is applied in the field of preparation of biomedical engineering materials with multiple functional groups on the surface, can solve problems such as difficult to achieve co-immobilization of multiple biomolecules, and achieve inhibition of smooth muscle cell adhesion and proliferation and reaction conditions. Mild and easy to use, the effect of enhancing activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0015] A method for preparing a multifunctional implant device, the steps of which are:
[0016] A. Construction of organic thin films containing multi-type functional groups
[0017] Immerse the implanted device modified with plasma polyallylamine coating in a Tris buffer solution (pH=8.5) of 0.1 mg / ml gallic acid, react for 2 hours, and after fully cleaning, introduce amino groups, carboxyl, quinone.
[0018] B. Co-immobilization of aptamer, bivalirudin and VEGF
[0019] The implanted device containing multiple types of functional groups obtained in step A is soaked in a WSC solution with a concentration of 10 μg / ml polypeptide aptamer, and the WSC solution is composed of the following components: 9.76 mg / ml of 2-(N-morpholine ) ethanesulfonic acid (MES) buffer solution, 1mg / ml of 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide (EDC) and 0.24mg / ml of N-hydroxysuccinamide ( NHS). After the reaction is sufficient, rinse fully with PBS (phosphate buffer solution) and distill...
Embodiment 2
[0022] A method for preparing a multifunctional implant device, the steps of which are:
[0023] A. Preparation of organic thin films containing multi-type functional groups
[0024] Prepare a gallic acid-Tris solution with a pH value of 10 and a concentration of 8 mg / ml, add 8 mg / ml decanediamine and a 316L stainless steel sample, react for 12 hours, and after thorough cleaning, a surface containing amino groups, carboxyl groups, Quinone-based organic thin films with multiple functional groups.
[0025] B. Co-immobilization of CD34 antibody and endothelial growth factor (VEGF)
[0026] Soak the multi-type functional group organic film obtained in step A in the buffer solution of WSC (the same components as in Example 1) containing 1 μg / ml CD34 antibody at a pH value of 5, react at room temperature at 20°C for 12 hours, and then use Rinse thoroughly with PBS and distilled water, then soak in PBS buffer solution containing 100ng / ml VEGF with a pH value of 7.4, react at room t...
Embodiment 3
[0029] A method for preparing a multifunctional implant device, the steps of which are:
[0030] A. Preparation of organic thin films containing multi-type functional groups
[0031] Prepare a gallic acid-Tris solution with a pH value of 10 and a concentration of 10 mg / ml, add arginine at 10 mg / ml and use a cobalt-based alloy as a substrate, react for 24 hours, and after thorough cleaning, a surface containing Amino, carboxyl, quinone-based organic films containing multiple types of functional groups.
[0032] B. Co-immobilization of CD34 antibody and heparin
[0033] Soak the multi-type functional group organic film obtained in step B in a buffer solution containing 10 μg / ml CD34 antibody with a pH value of 5 WSC (the components are the same as in Example 1), and react at room temperature for 2 hours at 20° C. Rinse thoroughly with PBS and distilled water as in 7.4. Then soak in the buffer solution of WSC (the same components as in Example 1) containing 1 μg / ml heparin at ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com