Danhong injection quality control method
A quality control method, the technology of Danhong injection, applied in the direction of measuring devices, instruments, scientific instruments, etc., to achieve the effect of simple method, avoiding one-sidedness and good reproducibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
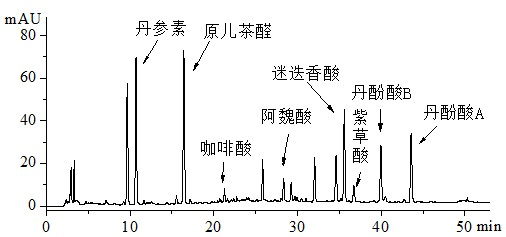
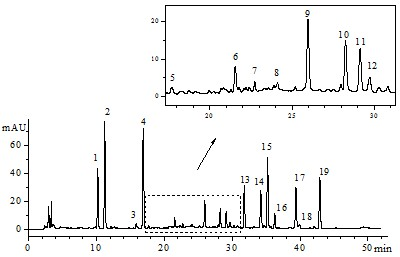
[0045] Embodiment 1: Danhong Injection Fingerprint Atlas Formulation
[0046] 1.1 Instruments and reagents
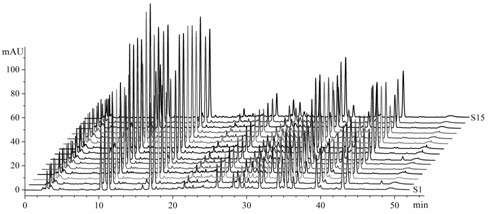
[0047] Agilent 1200 RRLC (including online vacuum degasser, high-pressure binary gradient pump, autosampler, column oven, diode array detector (DAD), chemstaion chromatography workstation); acetonitrile and formic acid are chromatographically pure; ultrapure water ; 15 batches of Danhong injection (Shandong Heze Buchang Pharmaceutical Co., Ltd., batch number (10mL): 101149, 101150, 110931, 110932, 110933, 110934, 110935, 110936, 101171, 101170, 101165, 101167, 101145, 101157, 10 ).
[0048] 1.2 Preparation of Danhong Injection Control Sample Solution
[0049] Precisely pipette 2 mL of Danhong injection, put it in a 10 mL volumetric bottle, dilute to the mark with acetonitrile-0.5% formic acid solution (50:50, v / v), shake well, and filter through a 0.45 μm microporous membrane , that is.
[0050] 1.3 Liquid chromatography conditions
[0051] Chromatographic conditio...
Embodiment 2
[0068] According to the method of Example 1, the difference is that the calculation of the correlation coefficient is used to evaluate the similarity of the fingerprints.
[0069] Compare the fingerprints of 15 batches of Danhong injection products to be tested with the established standard fingerprints, and calculate and identify the number of common peaks between the two. If the similarity is greater than 0.95, the injection is considered qualified; if the similarity is greater than 0.97, the injection is considered qualified. Liquid batches are stable and of good quality. It was found that for samples with a similarity > 0.990, the similarity obtained by the correlation coefficient algorithm was very close to the cosine value of the included angle, but the similarity values calculated by the samples with a low similarity were significantly different. See Table 3.
[0070] table 3
[0071]
Embodiment 3
[0073] According to the method of embodiment 1, the difference is to calculate the ratio qualitative similarity, i.e. the comparison fingerprint do sample do calculate and The cosine of the included angle. If the similarity is greater than 0.95, the injection is considered qualified, and if the similarity is greater than 0.97, the batch of injection is considered to be stable and of good quality. See Table 4 for the results.
[0074] Table 4
[0075]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com