Candesartan cilexetil and amlodipine tablet composition and preparation method thereof
A technology of candesartan cilexetil and amlodipine besylate, applied in the field of medicine, can solve the problems of reduced drug efficacy, increased amount of impurities, and poor stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Embodiment 1 Excipient compatibility test
[0040] Table 1
[0041]
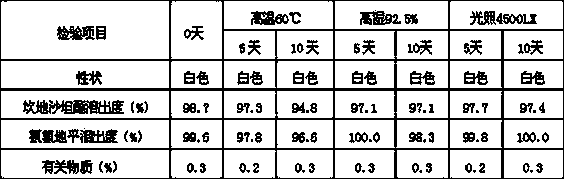
[0042] According to the results of related substances (maximum single impurity, total amount of impurities), except for PEG6000, other excipients have good compatibility with the two active ingredients, but the dosage of PEG6000 needs to be further screened.
Embodiment 2
[0043] The screening of embodiment 2 PEG dosage
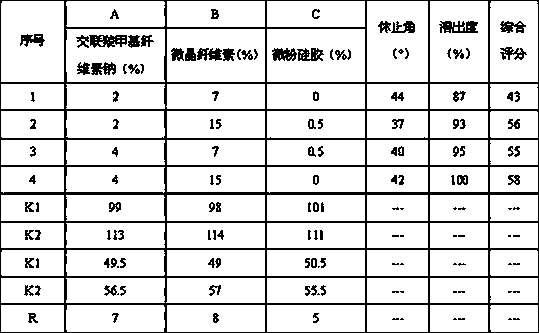
[0044] Primary selection of croscarmellose sodium 4%, microcrystalline cellulose 15%, micropowder silica gel 0.5%, magnesium stearate 1%, formula candesartan cilexetil besylate amlodipine (8 parts by weight / 6.93 parts by weight), the remaining mannitol 200SD supplements the total amount of the prescription to 100%, and the dosage of PEG6000 is screened by the results of related substances with an influencing factor of 60 degrees and 10 days.
[0045] Table 2
[0046]
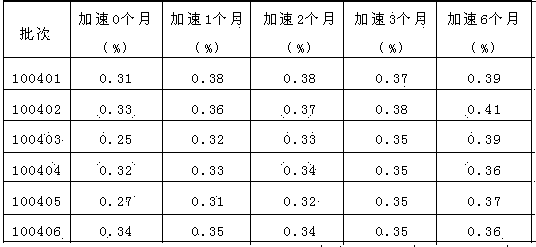
[0047] As can be seen from the results in Table 2, when the dosage range of PEG6000 is 2%-4%, the product impurity amount is low, and when the dosage is 3%, the impurity amount is the lowest, and the degradation products of candesartan cilexetil increase below this dosage range, and above this Amlodipine degradation products increased in the dosage range, and the total amount of impurities increased significantly.
Embodiment 3
[0048] Example 3 Prescription Screening
[0049] With reference to the situation of candesartan cilexetil tablets on the market, it is preliminarily determined that the tablet weight of the combination of candesartan cilexetil and amlodipine besylate is 0.130g.
[0050] Mannitol is non-hygroscopic and is an excellent diluent for moisture-sensitive active ingredients. Its dosage is adjusted to 100% according to the ratio of other excipients.
[0051] The 30-minute dissolution rate of candesartan cilexetil under the dissolution conditions in the quality standard is selected as the main index, and at the same time, the angle of repose reflects the fluidity of the particles, which has a certain relationship with the uniformity of the tablet, so the angle of repose of the mixed powder is secondary index. Since the smaller the angle of repose is better, its weight is set to -1, and the weight of the dissolution rate is 1, that is, the comprehensive score = dissolution rate (%) - an...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com