Method for detecting macromolecular substances in Shengqi Fuzheng injection
A technology for macromolecular substances and detection methods, applied in the field of drug analysis, can solve the problems of increasing decolorization steps, long operation time, complicated steps, etc., and achieves the effects of high detection sensitivity, guaranteed safety, and simple operation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Example 1: Detection of Injection Sample Macromolecular Substances
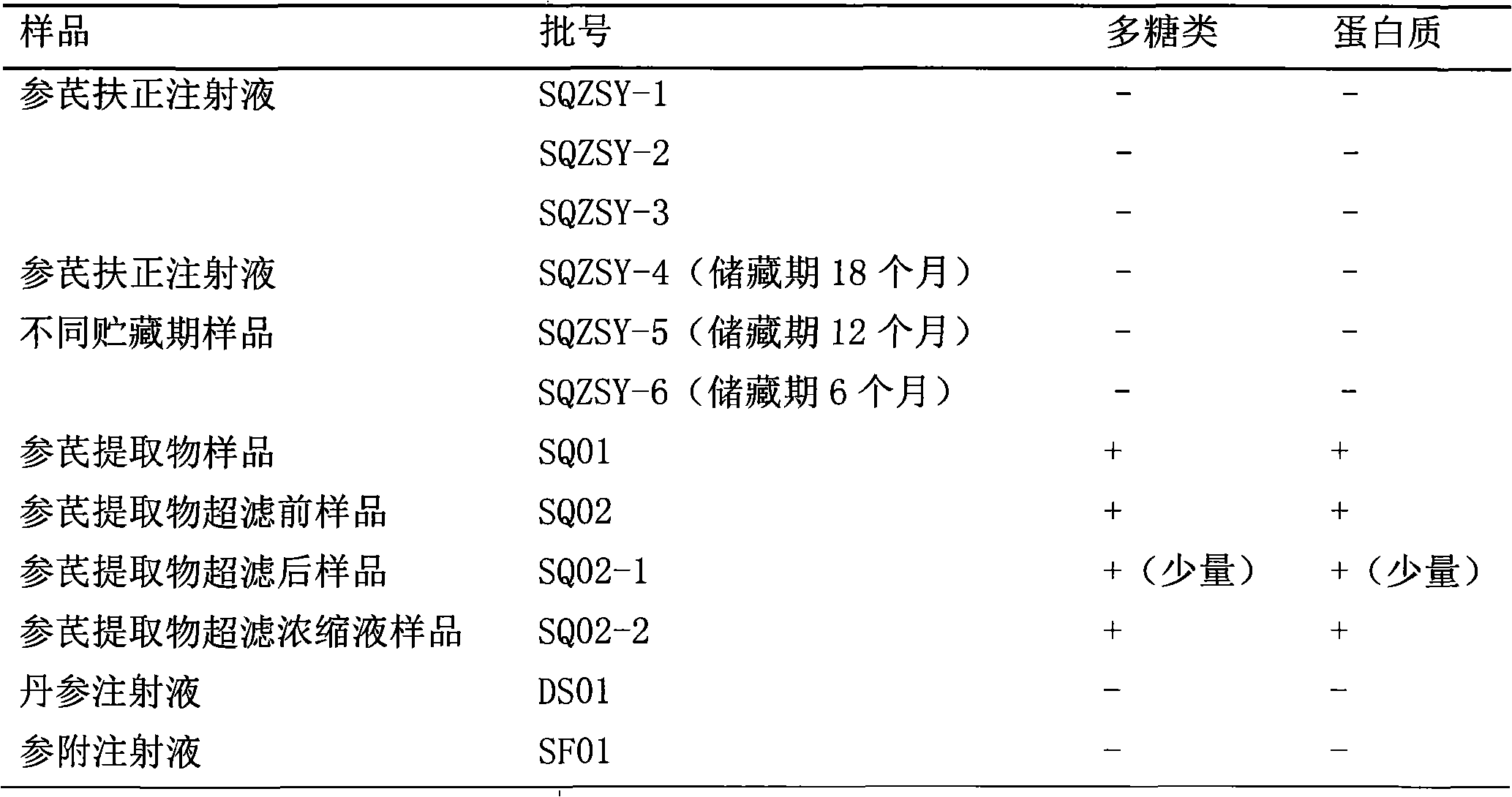
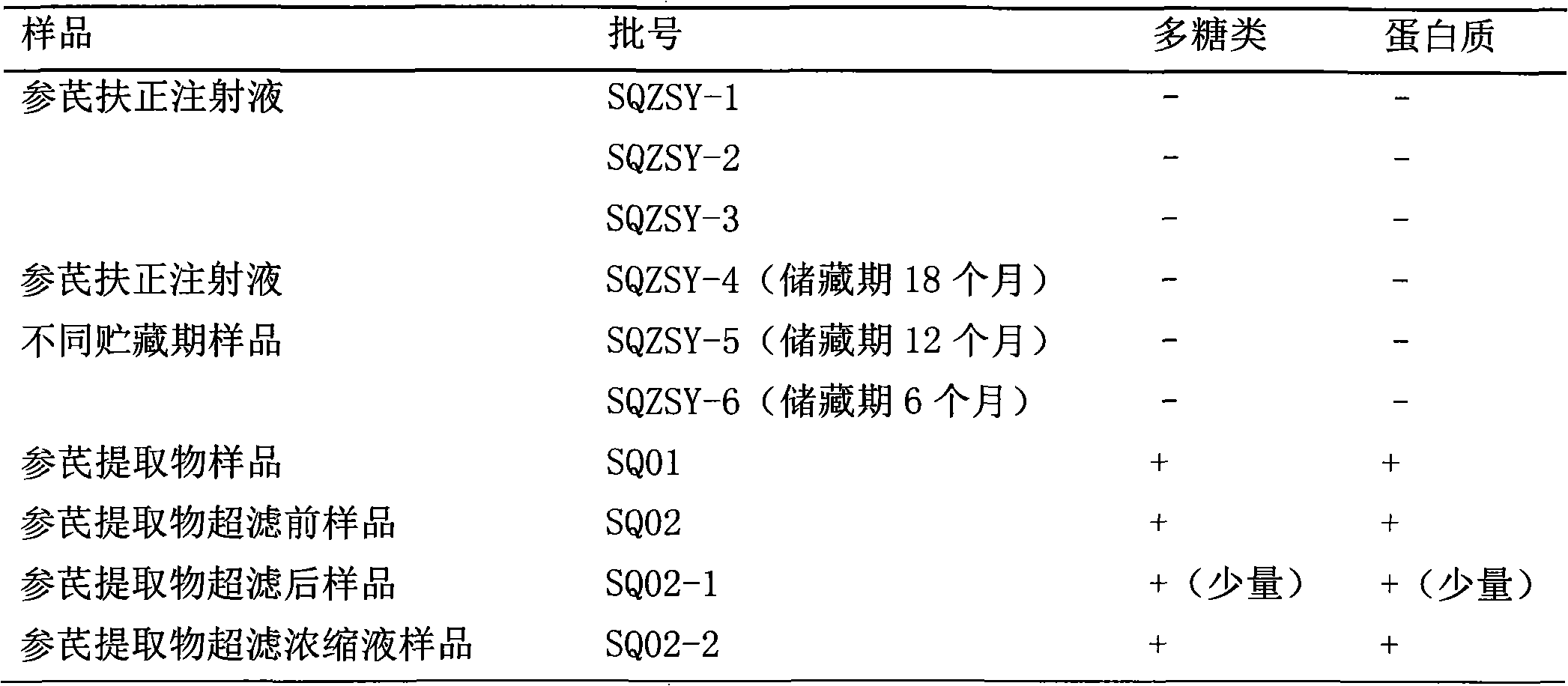
[0027] Precisely draw 10ml of each batch of Shenqi Fuzheng Injection, Danshen Injection, Shenfu Injection and each intermediate sample of Shenqi Fuzheng Injection into a Millipore Ultra-153.0kD ultrafiltration centrifuge tube, and centrifuge at 5000 rpm until the upper layer is retained Add 5ml of ultrapure water to the remaining 150μl of the retained solution in the upper layer, blow and mix with a pipette gun, centrifuge again until the remaining 150μl of the retained solution in the upper layer, add water repeatedly (8 to 10 times, 2ml each time), after adding water each time Mix well and then centrifuge until the part washed out from the lower layer is tested negative by Molish reaction, so as to ensure that it does not interfere with the detection of macromolecular substances. Collect the retentate in the upper layer, dilute it to 10ml, and use the BCA method and Molish method to detect whether it...
Embodiment 2
[0033] Example 2: Detection of injection sample macromolecular substances
[0034] Precisely draw 10ml of each batch of Shenqi Fuzheng Injection and Shenqi Fuzheng Injection intermediate samples, place them in Millipore Ultra-153.8kD ultrafiltration centrifuge tubes, centrifuge at 5000 rpm until the remaining 250 μl of the upper retentate, add ultra 5ml of pure water, after blowing and mixing with a pipette gun, centrifuge again until the remaining 250μl of the retained liquid in the upper layer, repeat adding water (8 to 10 times, 2ml each time), fully mix after each addition of water, and then centrifuge until the lower layer is washed. The out part was tested negative by Molish reaction to ensure that the detection of macromolecular substances was not interfered. Collect the retentate in the upper layer, dilute it to 10ml, and use the BCA method and Molish method to detect whether it contains macromolecular proteins and carbohydrates.
[0035] Molish reaction steps: add 1m...
Embodiment 3
[0042] Example 3: Allergy test on guinea pigs.
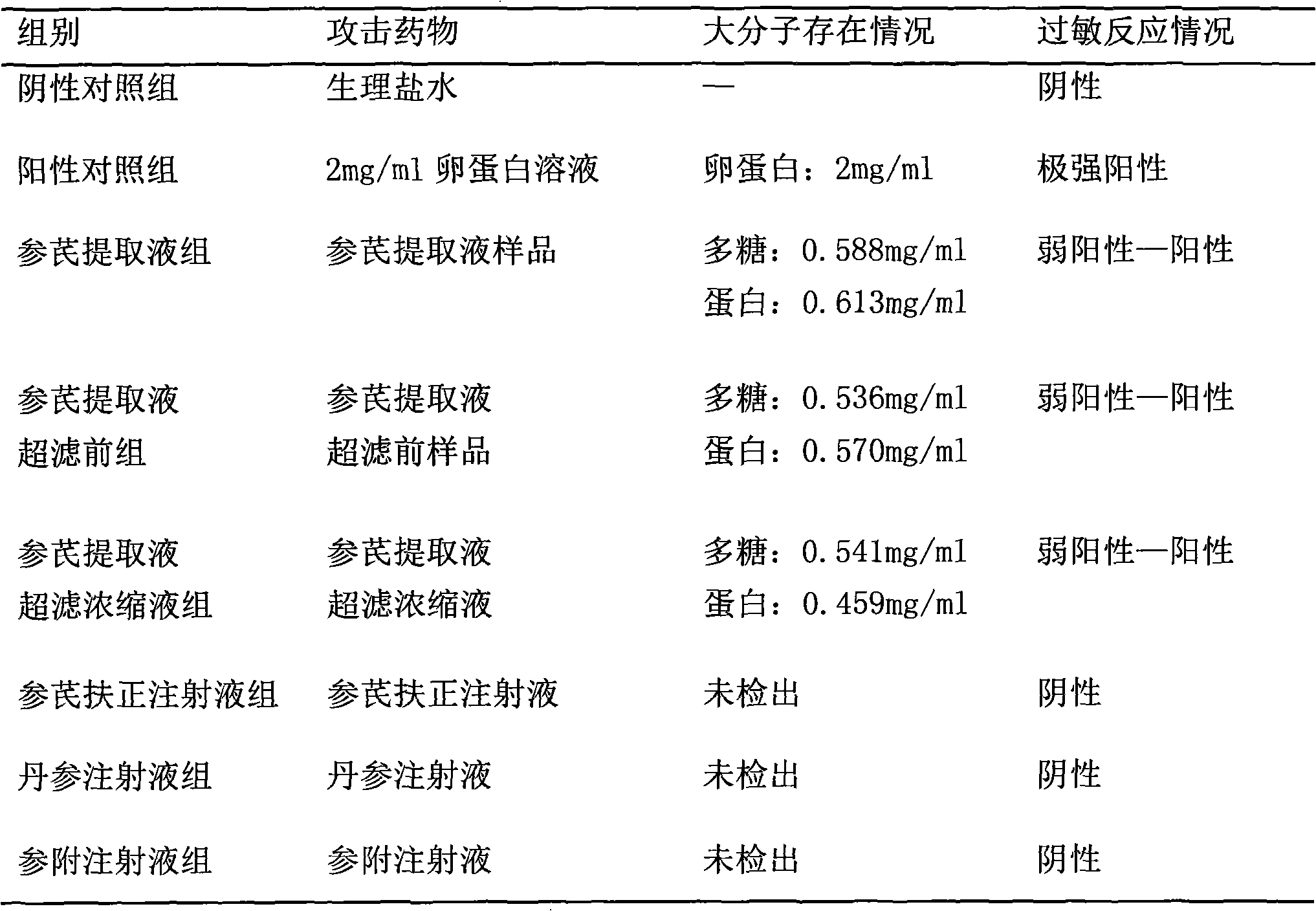
[0043] Using the method of establishing guinea pig systemic active allergy test animal model, the samples were tested and compared with the established method. Test according to the method of Pharmacopoeia Appendix XI K and "Technical Guidelines for Immunotoxicity (Allergy, Photosensitivity) Research of Traditional Chinese Medicine and Natural Medicines". Get healthy guinea pigs with a body weight of 250g to 350g and divide them into negative control group, positive control group, and each batch of intermediate samples (Shenqi extract sample, sample before ultrafiltration of Shenqi extract, sample of Shenqi extract ultrafiltration concentrate ) test group, Shenqi Fuzheng injection group, Danshen injection group, and Shenfu injection group, with 6 animals in each group. Each batch of Shenqi injection intermediate sample groups were converted into equivalent doses, and injected with a crude drug amount equivalent to 260mg / ml of S...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com