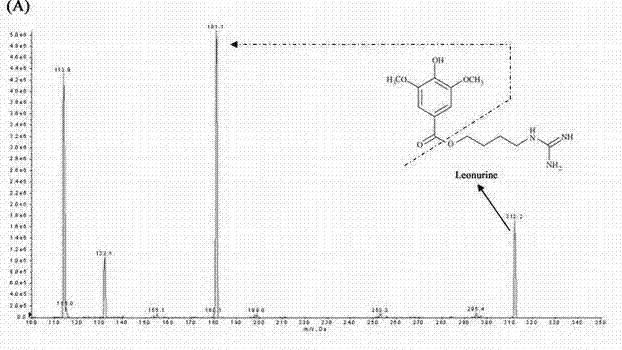
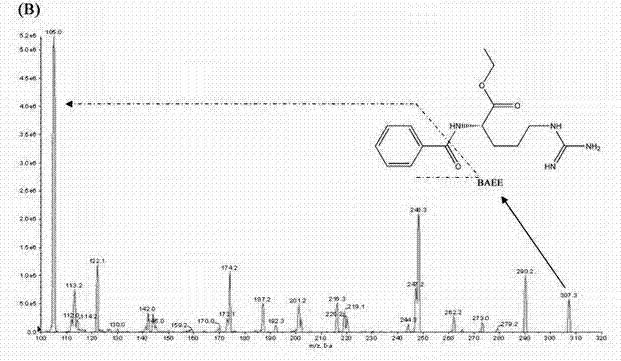
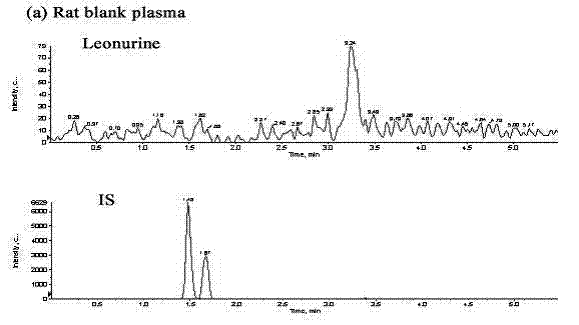
Method for quantitatively detecting content of leonurine in blood plasma
A technology for quantitative detection of leonurine, which is applied in the field of medical testing, can solve the problems of undetectable leonurine and low sensitivity, and achieve the effects of high sensitivity, strong specificity, and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Detection conditions and method examples
[0041] 1. Reagent preparation:
[0042] (1) Standard curve: Precisely prepare the motherwortine stock solution with deionized double-distilled water, the concentration is 1mg / mL, and then dilute it with double-distilled water to form different concentrations of the motherwortine standard working solution; take each concentration of the motherwortine standard working solution 10 μL, add 90 μL of blank rat plasma, and prepare standard curve solutions with concentrations of 4, 8, 16, 32, 64, 128, and 256 ng / mL respectively, a total of 7 concentration points, prepare 1 mL for each concentration, and store in aliquots At -80°C, to be tested;
[0043] (2) Quality control product: Take 10 μL of the standard working solution of motherwortine at an appropriate concentration, add 90 μL of blank rat plasma, and prepare four concentrations of motherurine with concentrations of 4, 10, 50, and 200 ng / mL as quality controls. Prepare 2 mL of...
Embodiment 2
[0073] Embodiment 2. Methodological verification:
[0074] Using the detection conditions and methods listed in Example 1, the assay method was validated methodologically. In the concentration range of 4-256ng / mL motherwortine, the linear relationship is good (r>0.995), the lower limit of quantification is 4ng / mL, and the lowest detection limit can reach 2ng / mL. The lower limit of quantitation precision is less than 20%, the other quality control intra-assay and inter-assay precisions are less than 15%, the accuracy is 98.73-105.42%, the recovery rate of motherwort extraction is 66.2-69.98%, RDS<10%, internal standard extraction The recovery rate was 62.78%, the RSD was 6%, the matrix effect of leonurine was 79.3–88.49%, and the matrix effect of the internal standard was 88.32%. Good stability. The results of method validation show that the established LC-MS / MS can quickly, sensitively, accurately and reliably detect the blood concentration of leonurine in rats administered ...
Embodiment 3
[0099] Detection of plasma concentration and calculation of pharmacokinetic parameters after oral administration of leonurine in rats.
[0100] Take 6 healthy male SD rats, take the blank blood before administration, and then give 60mg / kg intragastric administration, and then at 0.083, 0.167, 0.333, 0.5, 0.75, 1, 1.5, 2, 4, 8, 12 h Blood was collected and centrifuged to obtain plasma, and then the concentration of leonurine in the blood sample was measured according to the method of Example 1, and its pharmacokinetic parameters were calculated using the statistical moment method using DAS2.0 software (as shown in Table 6).
[0101] Table 6
[0102] Pharmacokinetic parameters unit Mean±S.D. Cmax μg / L 126.32±63.69 Tmax h 0.95±0.11 AUC(0-t) μg / L*h 411.60±147.96 AUC(0-∞) μg / L*h 432.87±146.72 MRT(0-t) h 3.21±0.44 MRT(0-∞) h 4.07±0.91 t 1 / 2 z h 2.77±0.61 CLz / F L / h / kg 151.54±49.05 Vz / F L / kg 622.83±289....
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| length | aaaaa | aaaaa |
| recovery rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com