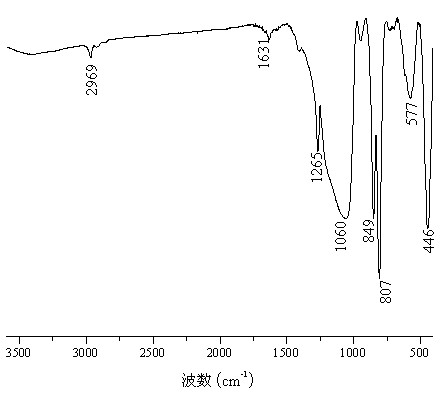
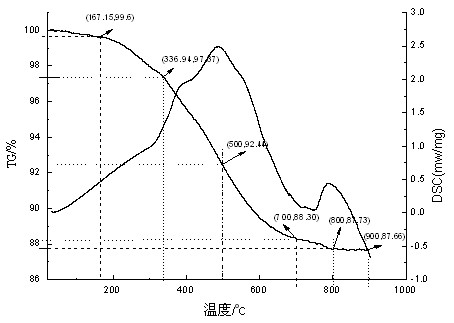
Synthesizing method for organic silicon micro-balls with performances of super hydrophobicity and high temperature resistant
A technology of organosilicon microspheres and synthesis methods, which is applied in the field of organosilicon synthesis, can solve problems such as large differences in reactivity, irregular product shapes, and non-environmental protection in the preparation process, and achieve the goal of overcoming large differences in reaction rates, regular shapes, The effect of excellent heat resistance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Under nitrogen protection, 32ml of tetramethyl orthosilicate, 21ml of hexane and 26ml of distilled water join in In the reaction bottle, react at 25°C for 0.5 hours. Add 27ml of dichlorodimethylsilane dropwise to the reaction system at a rate of 0.03ml / s, and then react for 1.0 hour after the dropwise addition, the temperature rises to 50°C, continue the reaction for 2.0 hours, add 20ml of silane coupling agent KH570 After reacting for 1.0 hour, it was precipitated by alcohol or acetone, filtered by suction, and dried to obtain organosilicon microspheres.
Embodiment 2
[0028] Under nitrogen protection, 44ml of tetraethyl orthosilicate, 35ml of hexane and 15ml of distilled water join in In the reaction bottle, react at 25°C for 1.0 hours, add 24ml of dichlorodiethylsilane dropwise to the reaction system at a speed of 0.45ml / s, and react for 2.0 hours after the dropwise addition, the temperature rises to 50°C, and continue the reaction for 1.5 hour, add 18ml of silane coupling agent A151 and react for 1.0 hour, precipitated by alcohol or acetone, filtered by suction, and dried to obtain silicone microspheres.
Embodiment 3
[0030] Under nitrogen protection, 23ml of propyl orthosilicate, 30ml of hexane and 20ml of distilled water join in In the reaction bottle, react at 25°C for 1.5 hours, add 18ml of dimethyldiethoxysilane dropwise to the reaction system at a speed of 0.4ml / s, and react for 3.0 hours after the dropwise addition, the temperature rises to 50°C, continue After reacting for 1.0 hour, add 23ml of silane coupling agent KH560 and react for 3.0 hours, precipitate through alcohol or acetone, filter with suction, and dry to obtain silicone microspheres.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com