Dihydroarteannuin derivatives and application thereof
A technology of dihydroartemisinin and derivatives, applied in the directions of active ingredients of heterocyclic compounds, medical preparations containing active ingredients, antibacterial drugs, etc., to achieve the effect of enhancing antibacterial efficacy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
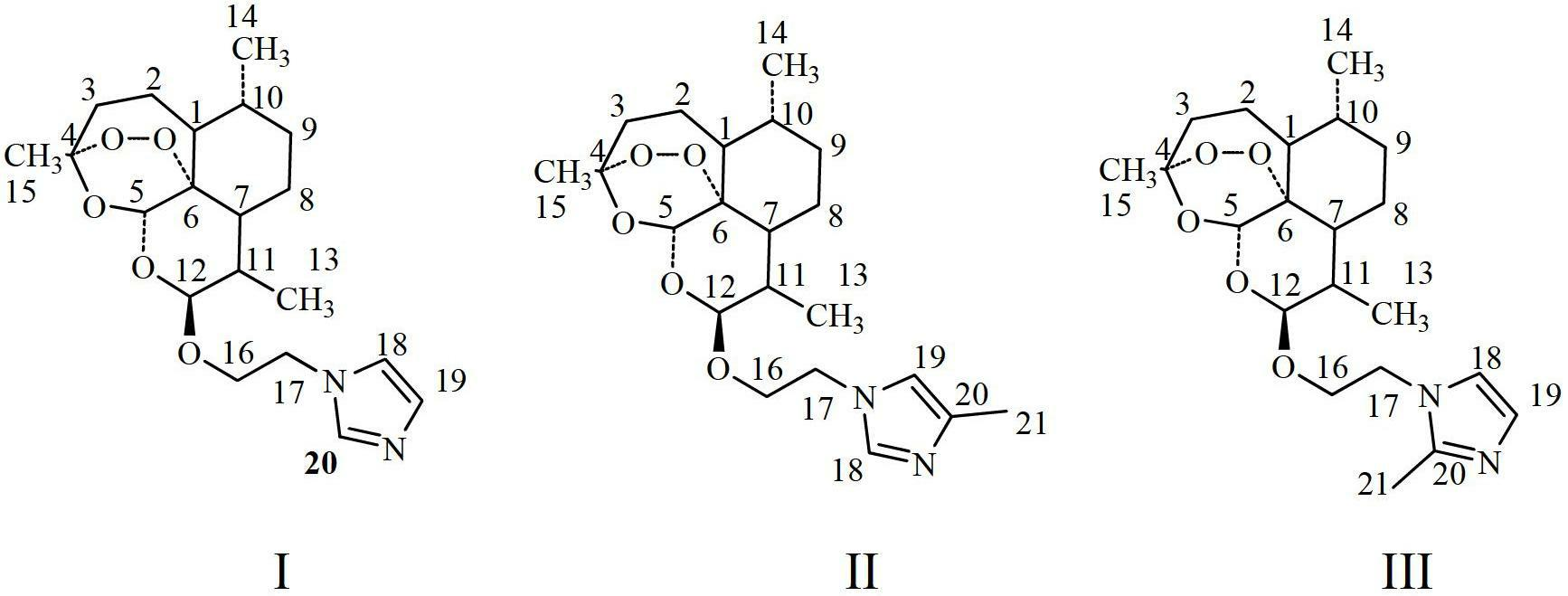
[0023] In this example, taking preferred compounds as examples, dihydroartemisinin ethoxyimidazole derivatives IED (4k), 4MIED (4l), and 2MIED (4m) were chemically synthesized. Now in conjunction with the following synthetic route for specific description.
[0024]
[0025] Preparation of 12β-(2-bromoethoxy)dihydroartemisinin (3):
[0026] In a 250mL round bottom flask, add 2-bromoethanol (2) 3.103g (24mmol) and Et 2 O 100mL, add BF under ice-bath cooling 3 .Et 2 O 4.0mL, continue to stir and react for 1.5h, add dihydroartemisinin (1) 5.690g (20mmol), continue ice bath cooling and stirring reaction, TLC monitors the reaction process. After the reaction is completed, add saturated NaHCO 3 Terminate the reaction. Separate the layers, extract the aqueous layer with EtOAc (30mL x2), combine the organic phases, wash with saturated brine 40mL, anhydrous MgSO 4 After drying, the solvent was removed by rotary evaporation under reduced pressure. The crude product was recrystalli...
Embodiment 2
[0034] synthetic compound 1 HNMR, 13 The structure was identified by CNMR and HRMS, and further characterized by optical rotation.
[0035] After identification, the structures of the synthesized dihydroartemisinin ethoxyimidazole derivatives 4k, 4l, 4m are as follows:
[0036]
[0037] The data characterization of the above dihydroartemisinin ethoxyimidazole derivatives in the present invention is as follows:
[0038] 4k(IED): 12β-(2-(1H-imidazol-1-yl)ethoxy)dihydroartemisinin
[0039] Yield: 89.1%;Yellow oily; (c 1.1mg / mL, CHCl 3 ). 1 H NMR (300MHz, CDCl 3 )δppm: 0.85(3H,d,J=7.2Hz,H-14),0.94(3H,d,J=6.1Hz,H-13),1.44(3H,s,H-15),1.22-2.07( 10H,m,H-2,H-3,H-7,H-8,H-9,and H-10),2.32-2.38(1H,m,H-1),2.61-2.63(1H,m ,H-11),3.62-3.68(1H,m,H-16),4.15-4.21(3H,m,H-16and H-17),4.76(1H,s,H-12),5.11(1H, s,H-5).7.00(1H,s,H-19),7.09(1H,s,H-18),7.62(1H,s,H-20); 13 C NMR (75MHz, CDCl3 )δppm: 128.7(C-20), 123.1(C-19), 118.9(C-18), 104.1(C-4), 102.0(C-12), 87.8(C-5), 80.8(C-6 ),67.0...
Embodiment 3
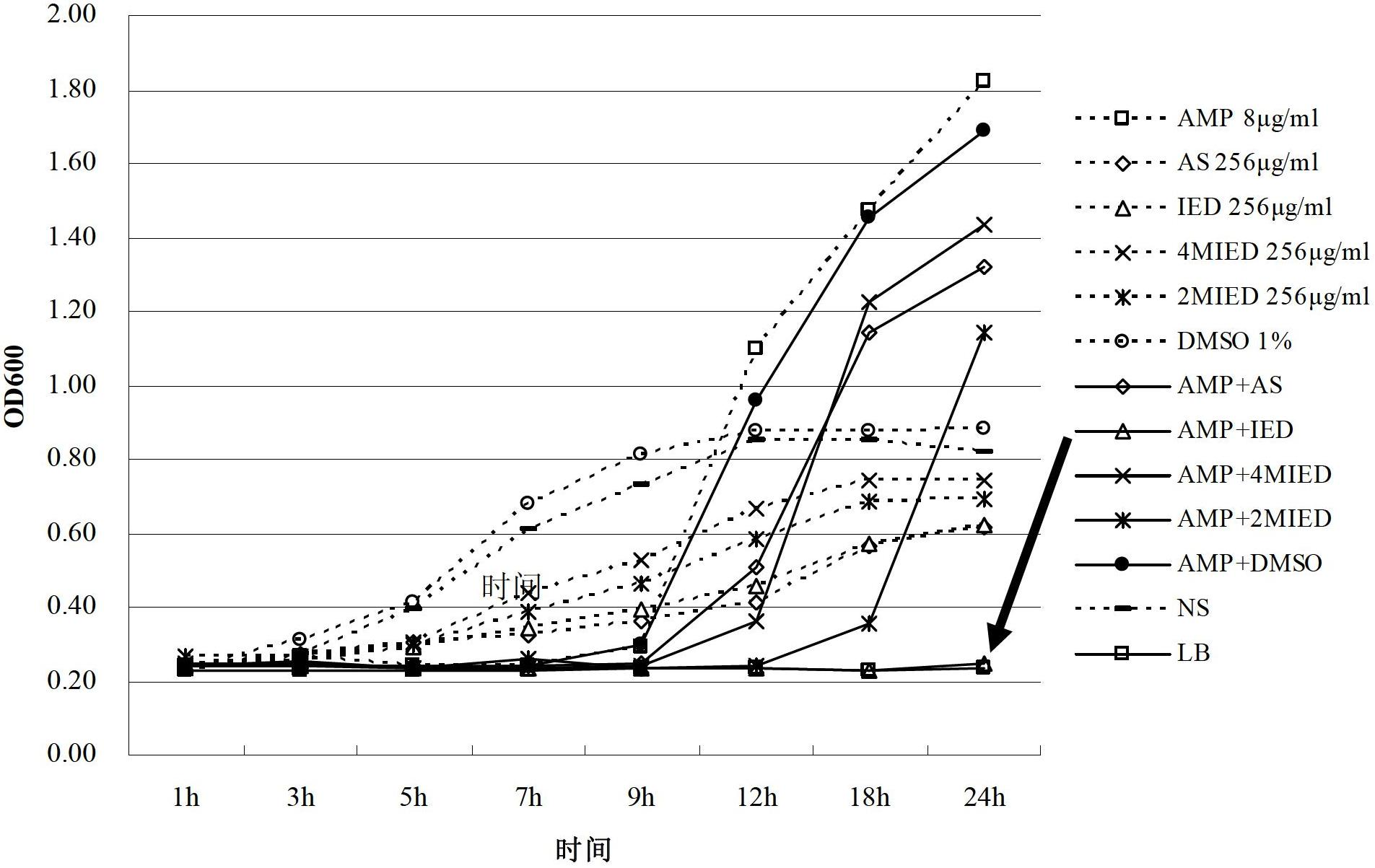
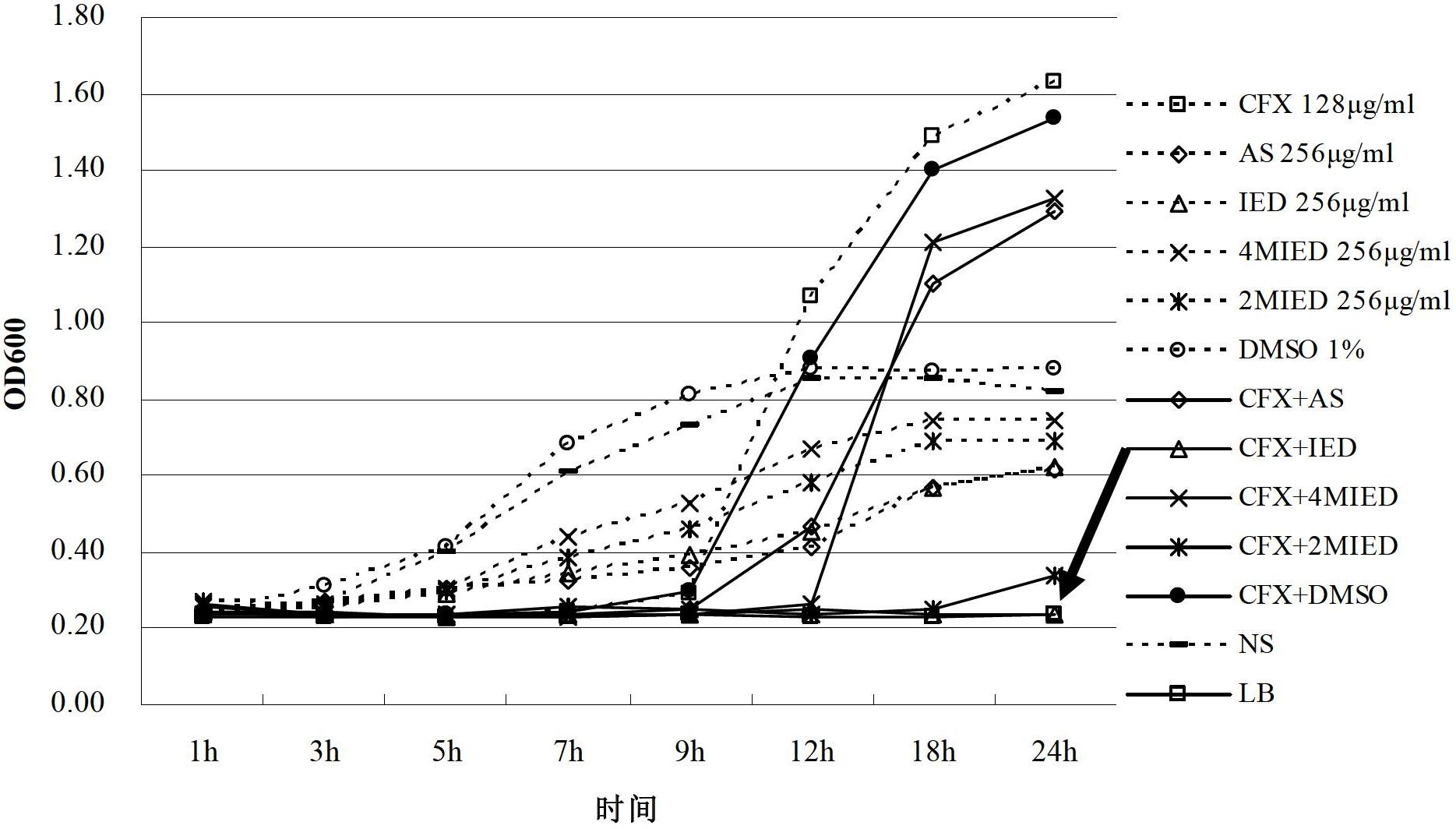
[0045] This experimental example is to study the effect of dihydroartemisinin derivatives and β-lactam antibiotics on the minimum inhibitory concentration (MIC) of Escherichia coli when used alone.
[0046] Using the microwell dilution method, adjust the bacterial concentration to 10 5 CFU / mL, inoculated in a 96-well sterile culture plate, dihydroartemisinin derivatives and β-lactam antibiotics ampicillin and cefuroxime were diluted with normal saline respectively. Add various drugs into the culture wells containing bacteria, and serially dilute, the final concentrations of the drugs in the 1st to 10th wells are 2048, 1024, 512, 256, 128, 64, 32, 16, 8, 4, 2μg / mL . Incubate in a 37°C incubator for 24 hours, read the positive and negative control wells, the negative control wells are clear, and the positive control wells are turbid. The MIC of a drug on bacteria is the lowest drug concentration that inhibits the growth of bacteria visible to the naked eye after 24 hours. The...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com