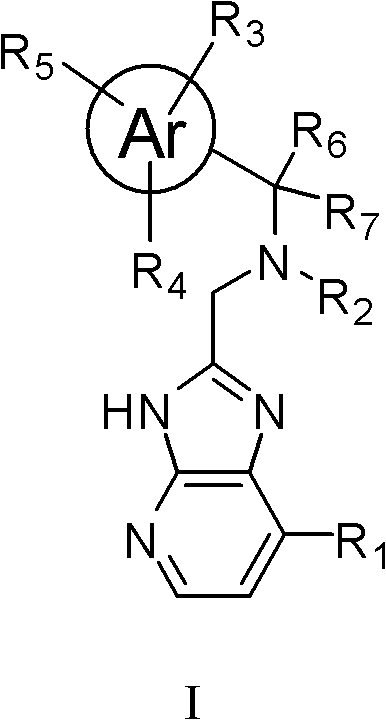
Imidazopyridine compounds, as well as preparation method and application thereof
A technology of imidazopyridine and compounds, applied in the field of imidazopyridine compounds, can solve the problems of low patient survival rate and poor prognosis of breast cancer patients
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0145] Compound 1: N 1 -((7-(4-methylpiperazin-1-yl)-3H-imidazo[4,5-b]pyridin-2-yl)methyl)-N 1 -((3-methylpyridin-2-yl)methyl)butane-1,4-diamine
[0146]
[0147] Step 1: 2-Amino-3-nitro-4-chloropyridine
[0148] Under stirring at 0°C, concentrated nitric acid (5.00 g, 0.0389 mol) and concentrated sulfuric acid (3.89 g, 0.0389mol), the addition was completed, and the mixture was naturally raised to room temperature and stirred for 1 hour. Then the reaction solution was poured into a mixture of 200 g of ice and 100 ml of water, and a large amount of yellow solids were precipitated, which were collected by filtration. The filtrate was neutralized with 28% ammonia water to pH 9, then extracted three times with ethyl acetate, and the organic phase was collected. The solid obtained by filtration was also dissolved in ethyl acetate, and the pH was adjusted to 9 with ammonia water, the separated organic phase was combined with the aforementioned organic phase, dried over anhyd...
Embodiment 2
[0172] Compound 2: N 1 -((7-(4-methylpiperazin-1-yl)-3H-imidazo[4,5-b]pyridin-2-yl)methyl)-N 1 -(pyridin-2-ylmethyl)butane-1,4-diamine
[0173] The reaction process was the same as in Example 1, except that 2-pyridinecarbaldehyde was used instead of 3-methyl-2-pyridinecarbaldehyde in step 7 to obtain a pale yellow gum.
[0174] 1 H NMR (300MHz, CDCl 3, ppm): δ8.56 (d, 1H, J = 4.2Hz), 7.96 (d, 1H, J = 6.0Hz), 7.54 (t, 1H, J = 7.5Hz), 7.33 (d, 1H, J = 7.8Hz), 7.11(t, 1H, J=6.0Hz), 6.35(d, 1H, J=5.7Hz), 5.58(brs, 2H), 3.89(s, 2H), 3.88(s, 4H), 3.78 (s, 2H), 2.61-2.53 (m, 8H), 2.32 (s, 3H), 1.57-1.50 (m, 2H), 1.45-1.41 (m, 2H); EI-MS: 408 (M) +
Embodiment 3
[0176] Compound 3: N 1 -(2-Methylphenyl)-N 1 -((7-(4-methylpiperazin-1-yl)-3H-imidazo[4,5-b]pyridin-2-yl)methyl)butane-1,4-diamine
[0177] The reaction process was the same as in Example 1, except that 2-methylbenzaldehyde was used instead of 3-methyl-2-pyridinecarbaldehyde in step 7 to obtain a colorless gum.
[0178] 1 H NMR (300MHz, CDCl 3 , ppm): δ7.91 (brs, 0.5H, imidazole NH), 7.80 (d, 1H, J=5.7Hz), 7.39 (brs, 0.5H, imidazole NH), 7.32 (d, 1H, J=7.2Hz ), 7.07-6.95(m, 3H), 6.29(d, 1H, J=5.7Hz), 5.41(brs, 2H), 3.90(s, 4H), 3.78(s, 2H), 3.60(s, 2H) , 2.71(s, 2H), 2.59(s, 4H), 2.46(s, 2H), 2.34(s, 3H), 2.24(s, 3H), 1.56(s, 4H); ESI-MS: 422.6(M +H) +
PUM
| Property | Measurement | Unit |
|---|---|---|
| pore size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com