Solid preparation containing amlodipine and valsartan
A solid preparation, amlodipine technology, applied in the field of pharmacy, can solve the problems of low drug loading, complex preparation process, low bioavailability of insoluble drugs, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
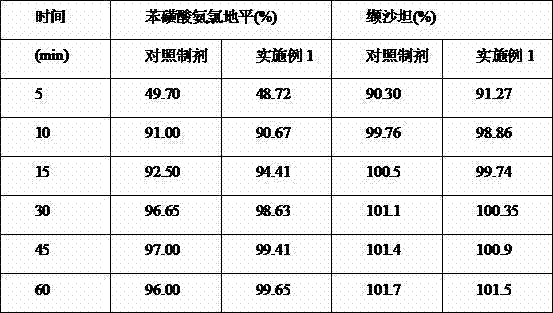
Embodiment 1
[0035] Material name Weight ratio (%) Amlodipine besylate (average particle size: 10μm) 1.99 Valsartan (average particle size: 60μm) 46.11 Microcrystalline Cellulose 101 (Filler) 24.50 Croscarmellose (disintegrant) 2.88 Hypromellose (binder) 2.31 Microcrystalline Cellulose 102 (disintegrant) 15.29 Magnesium Stearate (Lubricant) 1.18 Micropowder silica gel (glidant) 0.86
[0036] Table 1
[0037] Method: Amlodipine besylate and valsartan were pulverized through a 120-mesh sieve, and the corresponding prescription amounts of microcrystalline cellulose 101, hypromellose and croscarmellose were mixed evenly, and then mixed with 5% hypromellose Soft material made of cellulose aqueous solution, pass through 18 mesh sieve, control drying temperature at 50°C, granule moisture within 3.0%, granulate with 24 mesh sieve, weigh, add microcrystalline cellulose 102, micropowder silica gel and stearin in proportion Magnesium aci...
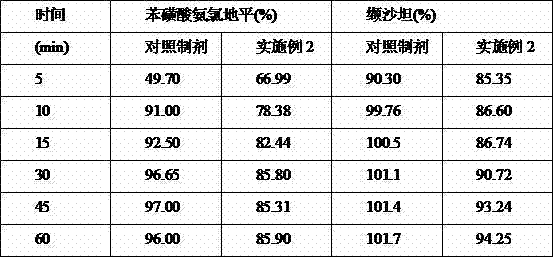
Embodiment 2
[0043] Material name Weight ratio (%) Amlodipine besylate (average particle size: 5μm) 1.99 Valsartan (average particle size: 55μm) 46.11 Microcrystalline Cellulose 101 (Filler) 28.38 Croscarmellose (disintegrant) 2.88 Hypromellose (binder) 3.31 Crospovidone K30 (disintegrant) 15.29 Magnesium Stearate (Lubricant) 1.18 Micropowder silica gel (glidant) 0.86
[0044] table 3
[0045] Method: Amlodipine besylate and valsartan were crushed through a 120-mesh sieve, and the corresponding prescription amount of microcrystalline cellulose 101 and croscarmellose were mixed evenly, and the soft material was made with 5% hypromellose aqueous solution. , through a 18-mesh sieve, control the drying temperature at 50°C, and keep the moisture content of the granules within 3.0%, sieve the granules with a 24-mesh sieve, weigh them, add microcrystalline cellulose 102, micronized silica gel and magnesium stearate in proportion, and ...
Embodiment 3
[0051] Material name Weight ratio (%) Amlodipine besylate (average particle size: 20μm) 1.99 Valsartan (average particle size: 10μm) 46.11 Microcrystalline Cellulose 101 (Filler) 24.50 Croscarmellose (disintegrant) 2.88 Hypromellose (binder) 2.31 Microcrystalline Cellulose 102 (disintegrant) 15.29 Magnesium Stearate (Lubricant) 1.18 Micropowder silica gel (glidant) 0.86
[0052] table 5
[0053] Method: Amlodipine besylate and valsartan were pulverized through a 120-mesh sieve, and the corresponding prescription amounts of microcrystalline cellulose 101, hypromellose and croscarmellose were mixed evenly, and then mixed with 5% hypromellose Soft material made of cellulose aqueous solution, pass through 18 mesh sieve, control drying temperature at 50°C, granule moisture within 3.0%, granulate with 24 mesh sieve, weigh, add microcrystalline cellulose 102, micropowder silica gel and stearin in proportion Magnesium aci...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com