Glutamine transaminase with improved enzymatic activity and thermal stability
A technology of glutamine and thermostability, which is applied in the field of transglutaminase, can solve the problems of poor thermostability, low specific enzyme activity, and lack of enzymes, and achieve the effect of reducing production costs and improving production efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Example 1: Simulation of MTG crystal structure derived from Streptomyces hygroscopicus
[0020] Using the reported TGase crystal structure of S.mobaraensis as a template, simulate the crystal structure of S.hygroscopicus TGase on the swiss-model website (http:∥swissmodel.expasy.org / ), as shown in figure 1 shown.
Embodiment 2
[0021] Example 2: Obtainment of highly active mutant strains (Del1-4)
[0022] 1. Use the site-directed mutagenesis kit (TaKaRa) to delete 7 amino acids (Asp 1, Ala 2, Ala 3, Asp 4, Glu 5, Arg 6, Val 7) at the N-terminus, and delete one amino acid named Del 1. By analogy, the deletion of the first seven amino acids was named Del1-7, and the transformant was sequenced by Shanghai Sangong.
[0023] 2. Transform the plasmid with correct sequencing into E.coli BL 21, select the transformant and inoculate it into LB liquid medium, culture at 37°C for 12 hours, and transfer it to TB medium with an inoculation volume of 3%. Bacteria grow to OD 600 When it was 2, IPTG was added to induce, and the culture temperature was lowered to 20°C, and cultured for 48h.
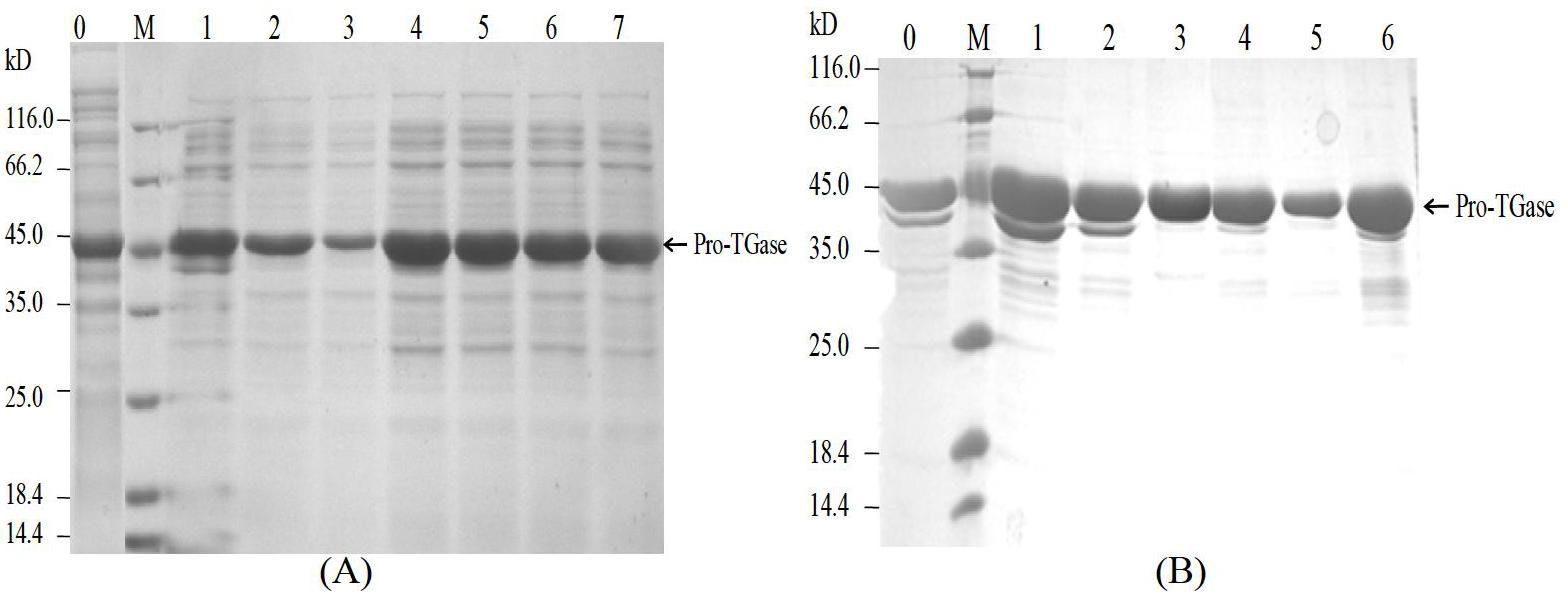
[0024] 3. Collect the fermentation supernatant, detect the enzyme activity of the fermentation supernatant, and purify the sample on a His-nickel column. The purified protein electrophoresis is as follows: figure 2 shown.
...
Embodiment 3
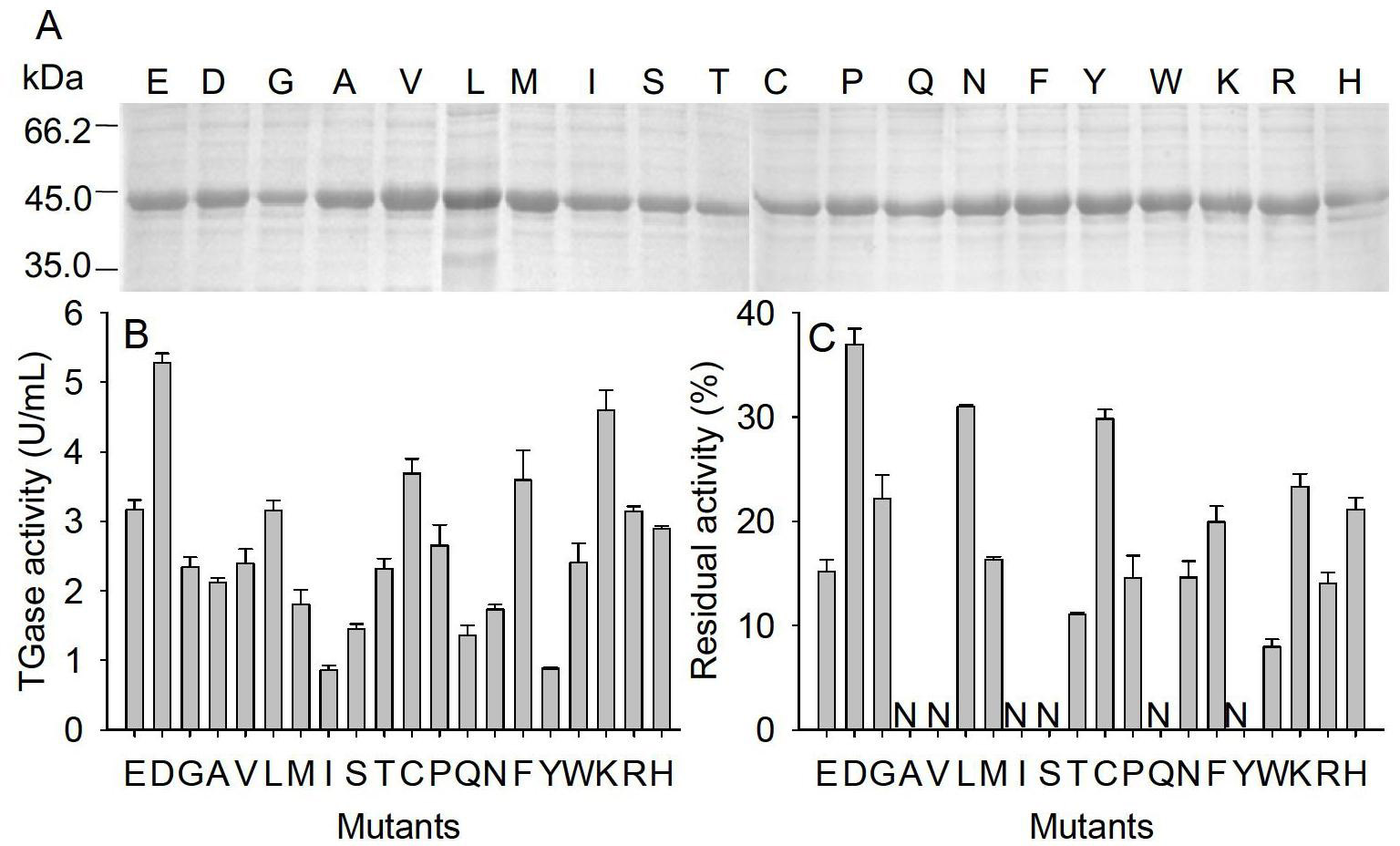
[0028] Example 3: Obtainment of highly active and thermostable mutants (TG-Del 1-4E5D)
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com