Construction method of in-vitro artificial scaffold protein mediated trehalose multienzyme complex
A complex and scaffolding technology, applied in the biological field, can solve the problems that have not been expanded to non-cellulase, and achieve the effects of reducing production costs, reducing waste, improving utilization and catalytic efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0151] Construction of recombinant strain WB800n-ScafCCR
[0152] (1) Clone the ScafCCR gene fragment
[0153] Using the ScafCCR bacterial liquid synthesized by Shanghai Sangong Company as a template, design primers for PCR amplification of the Rfcoh-Ctcoh-CBM-Cccoh gene fragment: using the Bacillus subtilis WB800n genome as a template, design primers for PCR amplification of the gene fragment of the P43 promoter, The gene fragment of phoD signal peptide, the gel electrophoresis picture is as follows Figure 5 shown;
[0154] The PCR primers of the ScafCCR protein gene sequence are as follows:
[0155] ScafCCR-F:5'-GGGGCCTTTGAAGTAATGACAACAACAGGCGGC-3'SEQ ID NO.10;
[0156] ScafCCR-R: 5'-CGACTCTAGAGGATCCTTAATGATGGTGATGATGATGTTGTGTGC-3' SEQ ID NO.11.
[0157] The PCR reaction system is as follows:
[0158] 2×Phanta Max Master Mix 25 μL, 10 μmol / L upstream primer ScafCCR-F 2.5 μL, 10 μmol / L downstream primer ScafCCR-R 2.5 μL, template 2.5 μL, use ddH 2 O supplemented to 50 ...
Embodiment 2
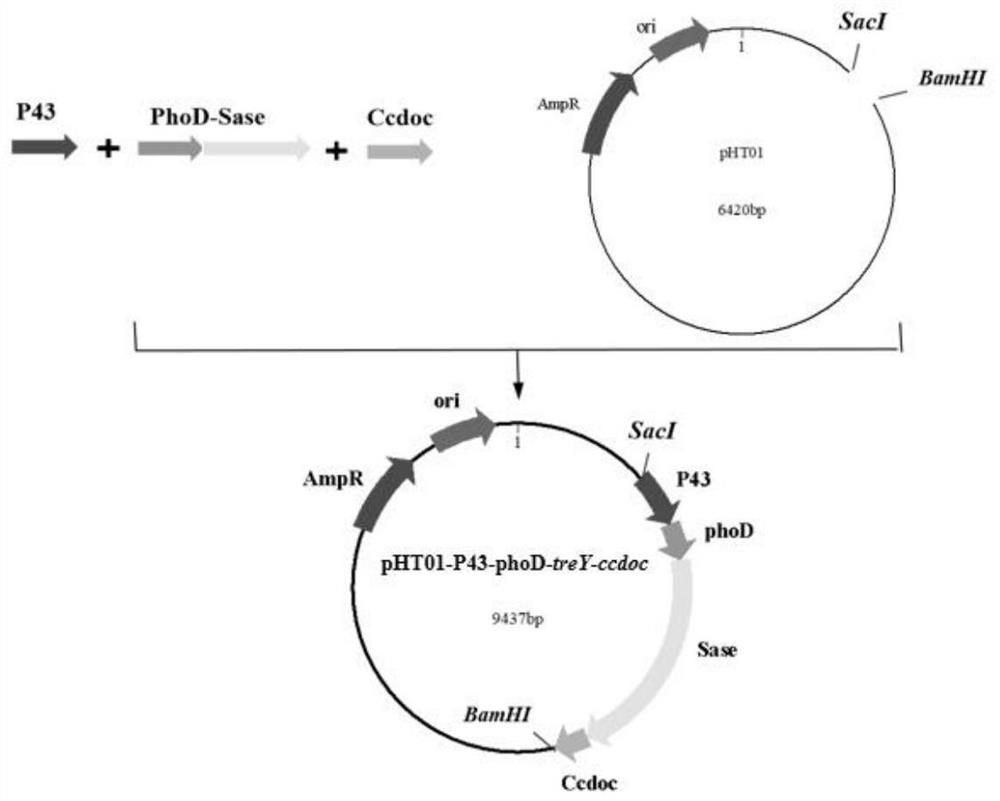
[0198] Construction of recombinant strain WB800n-P43-phoD-treY-Ccdoc
[0199] (1) Clone the treY and Ccdoc gene fragments of the maltooligosaccharyl trehalose synthase (MTSase) gene fragment
[0200] Using the genome of Bacillus subtilis WB800n as a template, primers were designed to amplify the gene fragment of the P43 promoter and the gene fragment of the phoD signal peptide by PCR, and the genome of Sulfolobus acidocaldarius ATCC 33909 was used as a template to amplify maltooligosaccharides by PCR Based on the trehalose synthase (MTSase) treY gene fragment, using the Sase2-Ccdoc bacterial liquid synthesized by Shanghai Sangong Company as a template, primers were designed for PCR amplification of the docking protein Ccdoc gene fragment, and the gel electrophoresis diagram is as follows Figure 5 shown.
[0201] The PCR primers of the P43 promoter gene sequence are as follows:
[0202] P43-F: 5'-AGTGAATTCGAGCTCAGCTTCGTGCATGCAGGCCGG-3'SEQ ID NO.6;
[0203] P43-R: 5'-TCAAAAC...
Embodiment 3
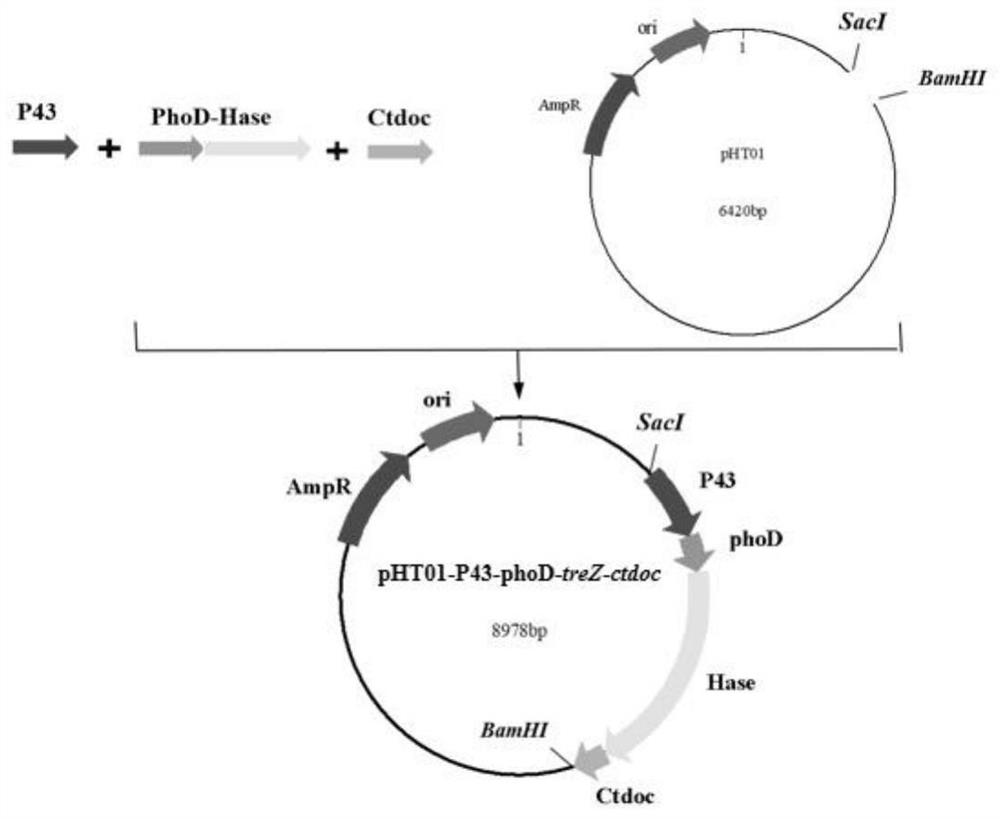
[0251] Construction of recombinant strain WB800n-P43-phoD-treZ-Ctdoc
[0252] (1) Clone the maltooligosaccharide-based trehalose hydrolase (MTHase) gene fragment treZ and Ctdoc gene fragment
[0253] Using the Escherichia coli strain P43-phoD-MTHase genome constructed in our laboratory as a template, primers were designed to amplify the gene sequence of phoD-maltooligosaccharide-based trehalose (MTHase) treZ by PCR. Template, design primers for PCR amplification of the docking protein Ctdoc gene fragment, the gel electrophoresis picture is as follows Figure 5 shown;
[0254] The PCR primers of the phoD-treZ gene sequence are as follows:
[0255] phoD-Hase-F:5'-GAATTAATAACAGAAGCTTATGGCATACGACAGTCGTTTTGATG-3'SEQ ID NO.17;
[0256] phoD-Hase-R: 5'-TGCCCGGAACTTTATACGTTTCTAATTGATATACCCCAACACCT-3' SEQ ID NO. 18.
[0257] The PCR reaction system is as follows:
[0258] 2×Phanta Max Master Mix 25 μL, 10 μmol / L upstream primer phoD-Hase-F 2.5 μL, 10 μmol / L downstream primer pho...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com