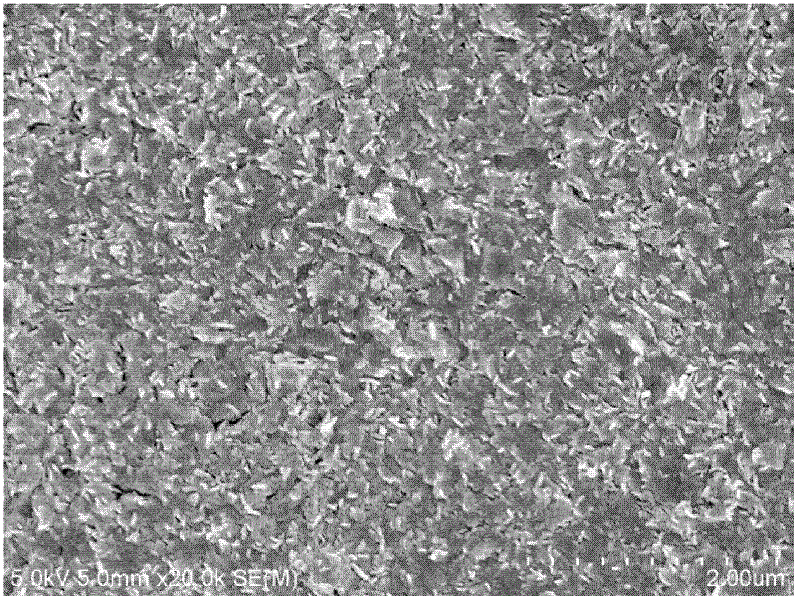
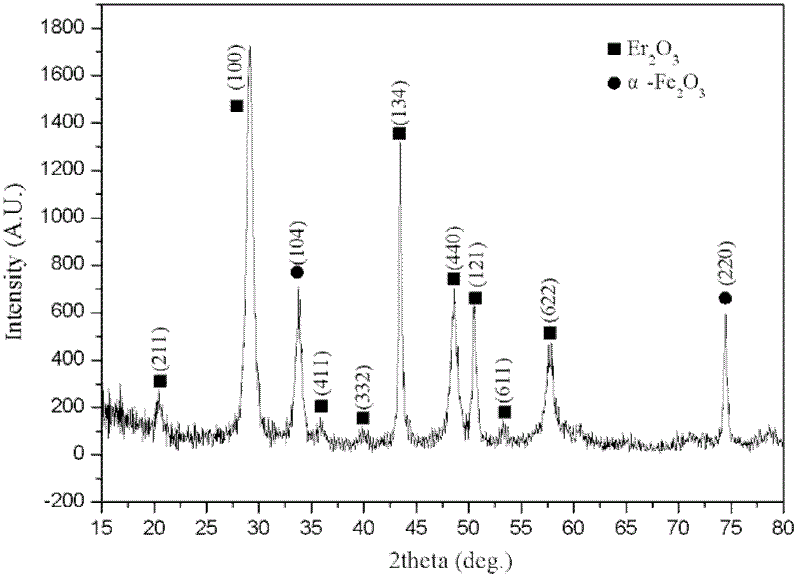
Method for preparing tritium resistance Er2O3 coating by using chemical solution method
A chemical solution method, er2o3 technology, applied in the field of preparation of tritium blocking coating, can solve the problem of high cost of tritium blocking coating, achieve the effect of low cost, high deposition rate and smooth coating
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] (1) Measure 20 mL and 1 mL of ethanol and terpineol respectively, and then mix the two;
[0024] (2) Stir the solution prepared in the above step (1) by a magnetic stirrer for 1 h at room temperature;
[0025] (3) add 0.0105mol erbium nitrate in the solution that above-mentioned step (2) makes, make the solution that metal Er ion concentration is 0.5mol / L;
[0026] (4) Stir the solution prepared in the above step (3) at 80° C. for 4 h with a magnetic stirrer to obtain a transparent light red erbium nitrate gel;
[0027] (5) the erbium nitrate gel prepared by step (4) is spin-coated on a 316L stainless steel substrate at a speed of 5000 rpm;
[0028] (6) The substrate coated with erbium nitrate gel in step (5) is placed in a high-temperature tubular quartz furnace, and 500 sccm argon gas is passed into the reaction chamber, and the temperature is raised to 600 ° C from room temperature at a heating rate of 5 ° C / h , and keep it warm for 3h, then the sample is cooled ...
Embodiment 2
[0030] (1) Measure 20mL and 1.5mL of ethanol and terpineol respectively, and then mix the two;
[0031] (2) Stir the solution prepared in the above step (1) by a magnetic stirrer at room temperature for 2 h;
[0032] (3) add 0.0172mol erbium nitrate in the solution that above-mentioned step (2) makes, make the solution that metal Er ion concentration is 0.8mol / L;
[0033] (4) Stir the solution prepared in the above step (3) at 85° C. for 6 h with a magnetic stirrer to obtain a transparent light red erbium nitrate gel;
[0034] (5) the erbium nitrate gel prepared by step (4) is spin-coated on a 316L stainless steel substrate at a speed of 6000 rpm;
[0035] (6) The substrate coated with erbium nitrate gel through step (5) is placed in a high-temperature tubular quartz furnace, and 500 sccm argon is passed into the reaction chamber, and the temperature is raised to 700 from room temperature with a heating rate of 5°C / h. ℃, and kept for 4h, then the sample was cooled to room te...
Embodiment 3
[0038] (1) Measure 20 mL and 2 mL of ethanol and terpineol respectively, and then mix the two;
[0039] (2) Stir the solution prepared in the above step (1) by a magnetic stirrer for 3 hours at room temperature;
[0040] (3) add 0.022mol erbium nitrate in the solution that above-mentioned step (2) makes, make the solution that metal Er ion concentration is 1.0mol / L;
[0041] (4) Stir the solution prepared in the above step (3) at 90°C for 8h with a magnetic stirrer to obtain a transparent light red erbium nitrate gel;
[0042] (5) the erbium nitrate gel prepared by step (4) is spin-coated on a 316L stainless steel substrate at a speed of 7000 rpm;
[0043] (6) Place the substrate coated with erbium nitrate gel through step (5) in a high-temperature tubular quartz furnace, feed 500 sccm argon into the reaction chamber, and heat up to 800 from room temperature with a heating rate of 5°C / h. ℃, and kept for 5h, then the sample was cooled to room temperature with the furnace, and E...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com