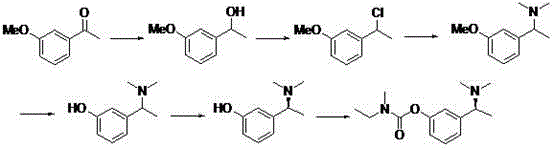
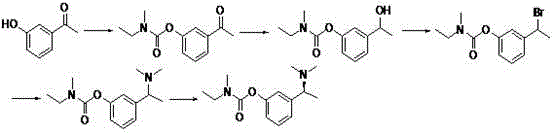
Synthetic method of anti-senile dementia medicinal rivastigmine racemic body
A technology of rivastigmine racemate and synthesis method, which is applied in chemical instruments and methods, preparation of organic compounds, preparation of carbamic acid derivatives, etc., can solve the problems of unsuitability for industrial production, complicated operation, high cost, etc., and achieve effective It is beneficial to industrial production and popularization and application, with high reaction yield and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
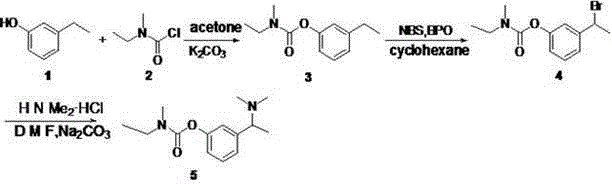
[0039] 1. Compound 3 preparation of
[0040] Add m-ethylphenol (12.2 g, 0.1 mol) to 130 ml of acetone, add potassium carbonate (41.5 g, 0.3 mol) and stir for 20 min, then add compound 2 (12.5 ml, 0.105 mol), refluxed for 7 hours. Cool to room temperature, vacuum-filter and evaporate acetone to dryness, add 30 ml of ethyl acetate, add 20 ml of water under ice bath, extract the water layer twice with ethyl acetate after separation, combine the ester layers, and dry over anhydrous sodium sulfate. Concentrate by filtration to obtain the compound 3 (19.706 g), yield 95.1%.
[0041] IR (KBr,cm -1 ) : 3433, 2968, 2934, 1724, 1478, 1399, 1236, 1164.
[0042] 1H NMR (400 MHz, CDCl3) δ 7.25 (t, J = 7.8 Hz, 1H), 7.07 – 6.86 (m, 3H), 3.52 – 3.33 (m, 2H), 3.04 (m, 3H), 2.65 (q, J = 7.6 Hz, 2H), 1.21 (m, 6H).
[0043] 13C NMR (101 MHz, CDCl3) δ 154.58, 151.45, 145.59, 128.91, 124.63, 121.07, 118.86, 43.95, 34.13, 33.69, 28.56, 15.23, 13.13, 12.40.
[0044] 2. Compound 4 preparation...
Embodiment 2
[0055] 1. Compound 3 preparation of
[0056] Add m-ethylphenol (12.2 g, 0.1 mol) to 130 ml of acetone, add sodium carbonate (15.2 g, 0.3 mol) and stir for 20 min, then add the compound dropwise 2 (12.5 ml, 0.105 mol), refluxed for 7 hours. Cool to room temperature, vacuum-filter and evaporate acetone to dryness, add 30 ml of ethyl acetate, add 20 ml of water under ice bath, extract the water layer twice with ethyl acetate after separation, combine the ester layers, and dry over anhydrous sodium sulfate. Concentrate by filtration to obtain the compound 3 (19.006 g), yield 91.8%.
[0057] 2. Compound 4 preparation of
[0058] compound 3 (2 g, 9.66 mmol) was dissolved in 40 ml cyclohexane, and NBS (3.44 g, 19.32 mmol) and AIBN (80 mg, 0.48 mmol) were added at 60 ° C, and the temperature was raised to 85 ° C, and then added every 20 min AIBN (80 mg, 0.48 mmol), AIBN was added three times. After refluxing for three hours, stop the reaction, spin dry, add ethyl acetate to di...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com