Method for preparing titanium white
A technology of titanium dioxide and ferro-titanium, applied in the direction of titanium dioxide, titanium oxide/hydroxide, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
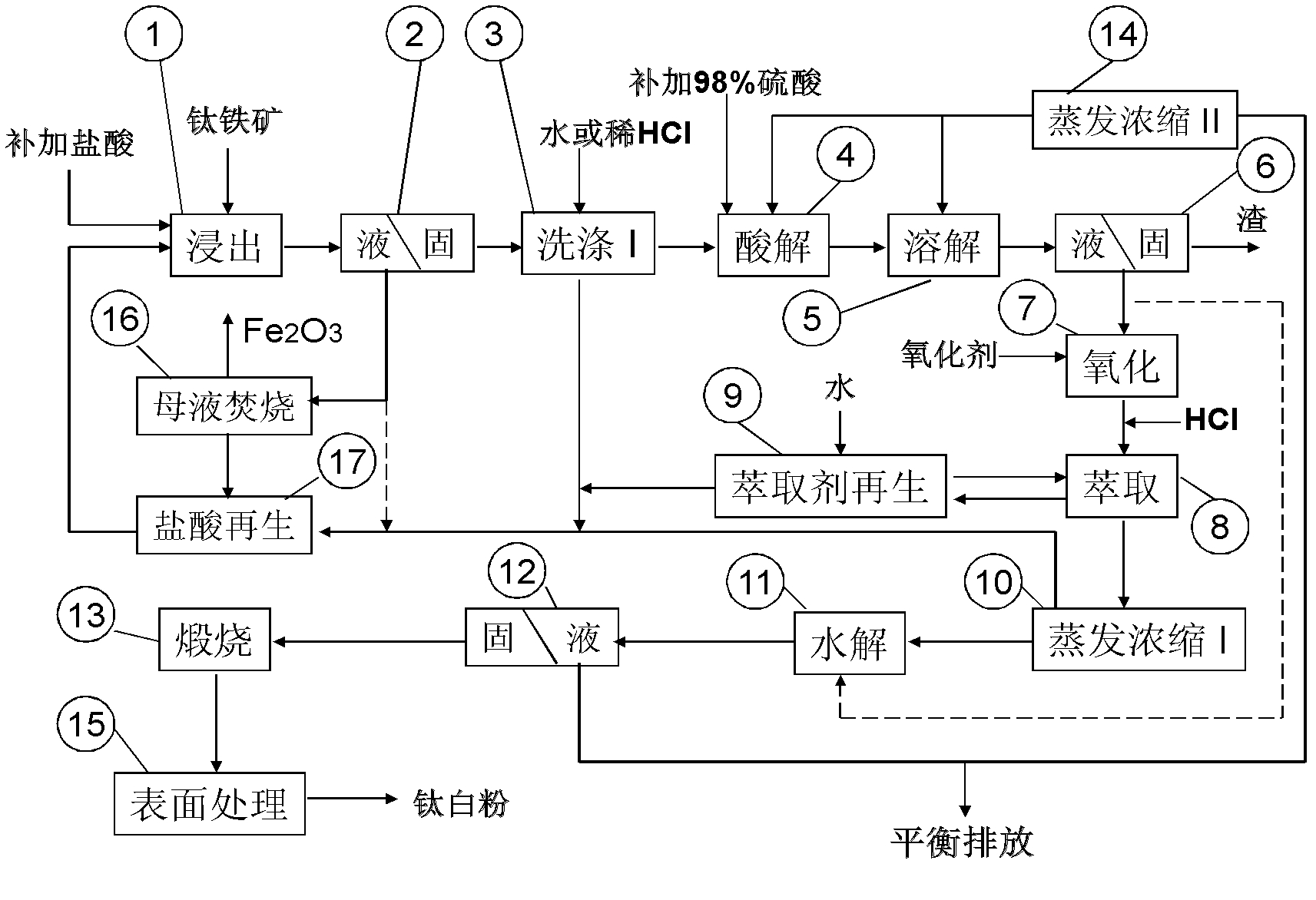
[0217] A. Direct leaching of titanium concentrate with hydrochloric acid to prepare high-titanium hydrochloric acid leaching slag
[0218] 1. Weigh 7.2 tons of titanium concentrate, pour it into the reaction tank, and seal it.
[0219] 2. Pump 25 cubic meters of hydrochloric acid leaching solution with a concentration of 19% in the reaction tank. The hydrochloric acid leaching solution contains TFe 42g / L; Ca 2+ 1.2g / L; Mg 2+ 6.7g / L; Al 3+ 0.8g / L; Mn 2+ 2.0g / L, the leachate is preheated to 100°C by a graphite heat exchanger.
[0220] 3. Rotate the reaction tank to fully mix the leachate in the reaction tank with the titanium concentrate.
[0221] 4. Put steam into the reaction tank, quickly heat the leaching solution to about 120°C, and stop the steam.
[0222] 5. The heat of reaction will raise the temperature of the reaction tank to about 135°C. After holding the leaching solution for 3 hours, stop the reaction tank and directly discharge the reaction slurry into the fla...
Embodiment 2
[0252] Steps A, B, and D are the same as in Example 1, and the extraction agent used for the purification of step C titanium liquid is different,
[0253] details as follows:
[0254] After adding hydrochloric acid in Step C of Example 1, the titanium solution was subjected to three-stage countercurrent extraction, and the organic phase was formed by mixing 30% methyl isobutyl ketone (TBP) and 70% sulfonated kerosene. The O / A ratio of 1:1 is selected for extraction, and ferric ions are selectively extracted into the organic phase to be removed. The chemical composition of the titanium solution after extraction is shown in Table 14.
[0255] Table 14, the chemical composition of titanium liquid after extraction
[0256]
[0257]
[0258] The extracted titanium solution was sprayed under negative pressure to remove hydrochloric acid and concentrated at a constant temperature of 80°C to obtain a high-purity titanium solution. Its chemical composition is shown in Table 15....
Embodiment 3
[0262] see Figure 4 , increase the reaction slurry classification process in step A of embodiment 1, discharge the fine-grained slurry in the upper layer of the reaction tank to cool in the flash tower, then filter and wash with dilute hydrochloric acid, and the filter cake is dried in a rotary kiln to obtain High titanium hydrochloric acid leaching residue. The coarse-grained slurry in the lower layer of the reaction tank is cooled and directly filtered for solid-liquid separation. The filter cake is washed with dilute hydrochloric acid, dried naturally, and then calcined at 950°C in a rotary kiln to obtain artificial rutile, which can be used in the production of titanium dioxide by the chloride method.
[0263] The high-titanium hydrochloric acid leaching residue prepared in Step A of Example 1 was further implemented in the same manner as Steps B, C, and D of Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com