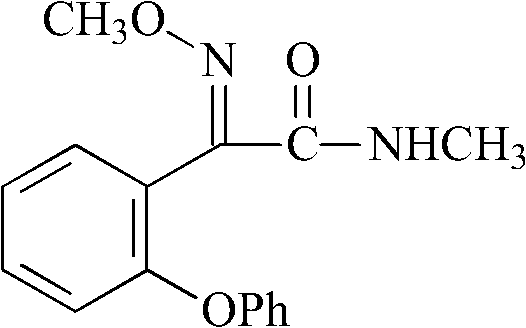
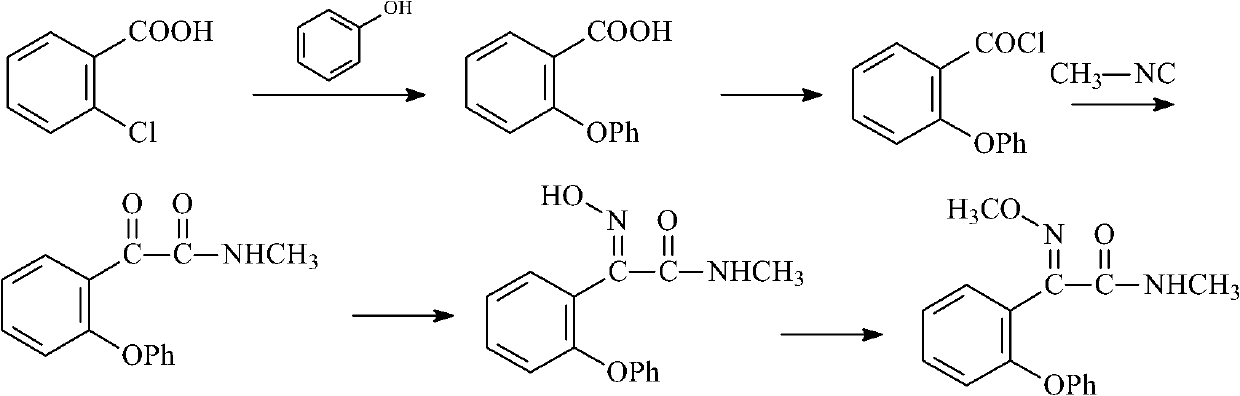
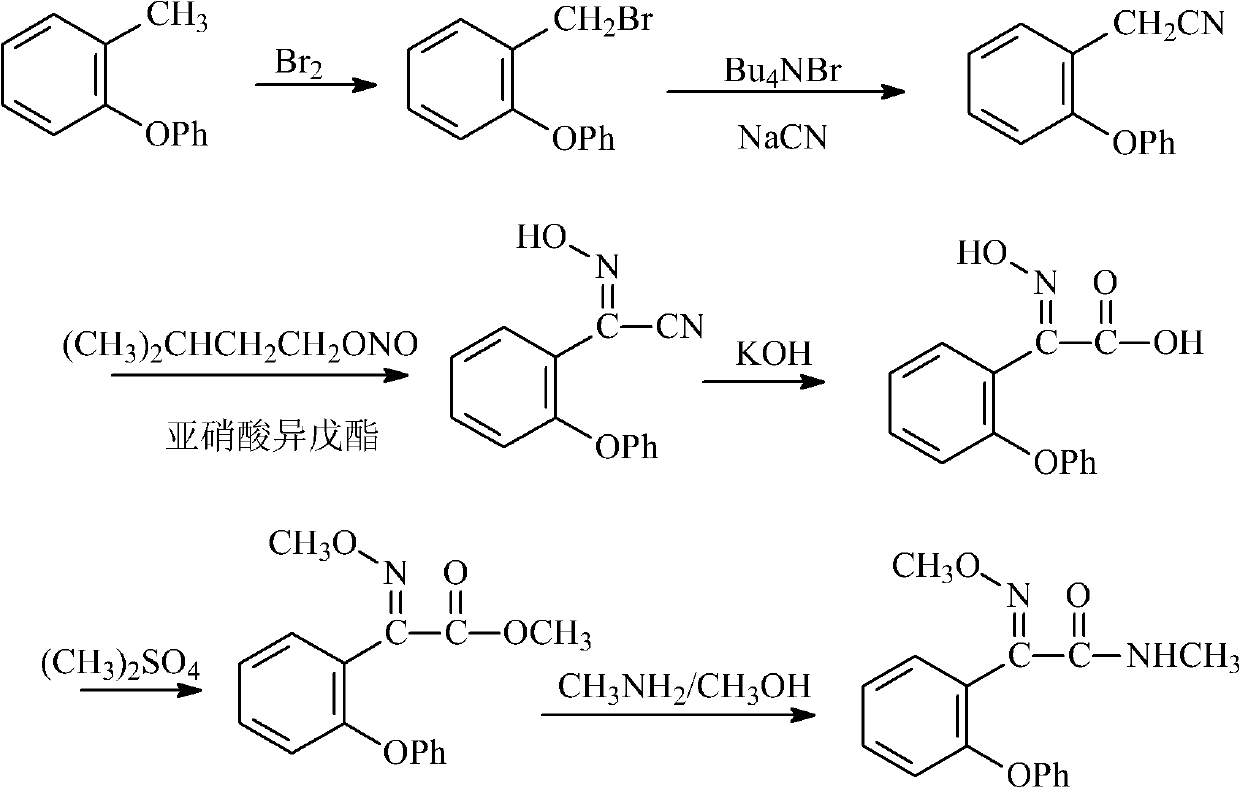
Preparation method of metominostrobin
A technology of phenoxystrobin and phenoxy, which is applied in oxime preparation, organic chemistry, etc., can solve the problems of operators and environmental hazards, unsuitability for industrial production, and high environmental pressure, and achieve shortened reaction time, less waste, and The effect of increasing the yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] (1) Synthesis of 2-(phenoxy)benzonitrile
[0039]
[0040] In a four-necked reaction flask equipped with a stirrer, a thermometer, a reflux condenser and a dropping funnel, add 15.5 g (0.3 mol) of ethanedinitrile and 150 ml of toluene, pour in hydrofluoric acid to saturation, and cool to the reaction temperature -20°C, add 51.0g (0.3mol) of diphenyl ether dropwise, after the dropwise addition, keep warm at this temperature for 3h, gradually rise to room temperature, stir overnight, pour the reaction mixture into 2kg of ice water, and then use 3× 150ml of dichloromethane for extraction. The organic phases were combined and concentrated to obtain phenoxybenzonitrile with a yield of 71%. The melting point is 45°C.
[0041] NMR (CDCl3) ppm: 6.92 (2H, dd), 7.11 (2H, m), 7.17 (1H, m) 7.22 (2H, m), 7.50 (1H, s), 7.77 (1H, s)
[0042] (2) Synthesis of 2-(2-phenoxybenzene)-2-oxoacetamide
[0043]
[0044] Add 240ml of 6N hydrochloric acid solution, 54g (0.24mol) of 2-(...
Embodiment 2
[0055] (1) Synthesis of 2-(phenoxy)benzonitrile
[0056]
[0057] In a four-necked reaction flask equipped with a stirrer, a thermometer, a reflux condenser and a dropping funnel, add 34.1g (0.66mol) of ethanedinitrile and 300ml of toluene, pour in hydrofluoric acid to saturation, and cool to the reaction temperature -15°C, add 102.0g (0.6mol) diphenyl ether dropwise, after the dropwise addition, keep warm at this temperature for 3h, gradually rise to room temperature, stir overnight, pour the reaction mixture into 4Kg of ice water, and then use 3×150ml Dichloromethane extraction. The organic phases were combined and concentrated to obtain phenoxybenzonitrile with a yield of 71.5%. The melting point is 45°C.
[0058] (2) Synthesis of 2-(2-phenoxybenzene)-2-oxoacetamide
[0059]
[0060] Add 480ml of 6N hydrochloric acid solution, 108g (0.48mol) of 2-(phenoxy)benzonitrile, and 4.32g (0.24mol) of water into a 1000ml four-necked reaction flask, stir at room temperature f...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| water solubility | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com