Flurbiprofen sustained release capsules and preparation method thereof
A flurbiprofen and sustained-release capsule technology, applied in the field of flurbiprofen sustained-release preparations, can solve problems such as poor safety, and achieve the effects of small differences in bioavailability, good fluidity, and reduced drug toxicity and side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
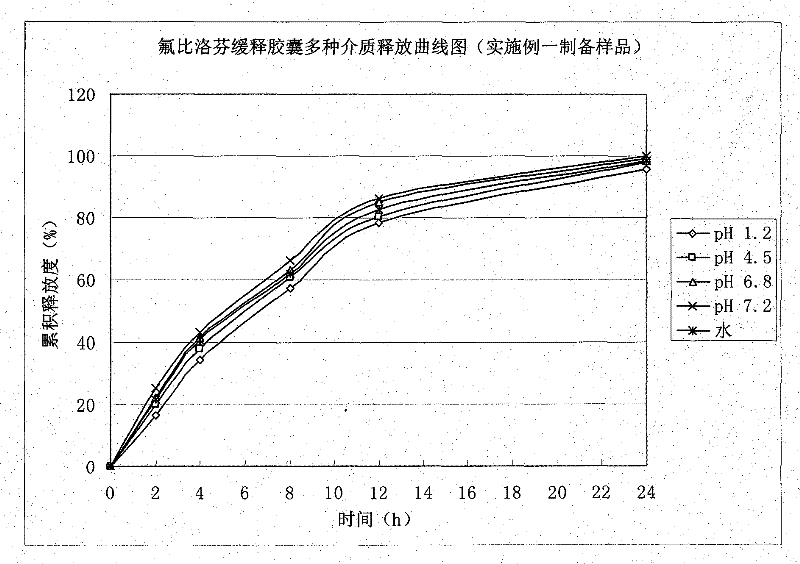
Embodiment 1
[0034] 1. Preparation of drug-containing pill core:
[0035]
[0036] Preparation process: mix flurbiprofen and microcrystalline cellulose uniformly in an equal increasing method, add sodium lauryl sulfate to 35% ethanol solution and dissolve to prepare sodium lauryl sulfate ethanol solution, mix the above The powder is made into a soft material, and pellets are extruded and spheronized. The particle size of the sieve plate is 0.8mm, oven-dried at 60°C, sieved, and 20-40 meshes containing pill cores are selected for coating.
[0037] 2. Package slow-release layer:
[0038]
[0039] Add the prescribed amount of Surelease into purified water, make it fully dispersed, stir for 45 minutes, place the drug-containing pellet core in a fluidized bed, adjust various process parameters to ensure a fluidized state, and increase the weight of the coating to 12%.
[0040] Process parameters of fluidized bed: Inlet air volume: 20~30HZ, Inlet air temperature: 45~55℃, Material temperat...
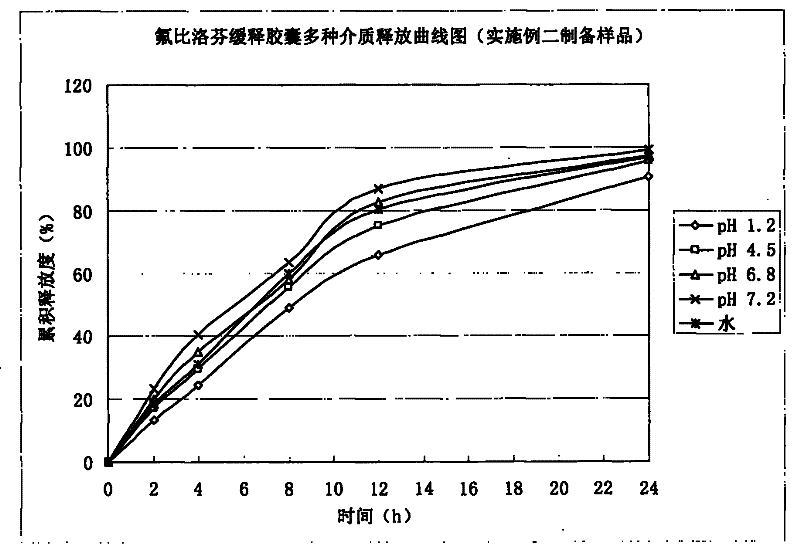
Embodiment 2
[0046] 1. Preparation of drug-containing pill core:
[0047]
[0048] Preparation process: Mix flurbiprofen, lactose, and microcrystalline cellulose uniformly in equal increments, add Tween into 35% ethanol solution, make the above mixed powder into a soft material, and use extrusion spheronization mechanism pellets , the particle size of the sieve plate is 0.8mm, oven-dried at 60°C, sieved, and 20-40 mesh-containing pill cores are selected for coating.
[0049] 2. Package slow-release layer:
[0050]
[0051] Add the prescribed amount of talc powder into purified water and stir for 45 minutes, add Eudragit NE30D water dispersion to make it fully dispersed, continue stirring for 45 minutes, and set aside. The pill core containing the drug is placed in a fluidized bed, and various process parameters are adjusted to ensure a fluidized state, and the weight of the coating is increased to 4%.
[0052] Process parameters of fluidized bed: air intake: 20-30HZ, air intake tem...
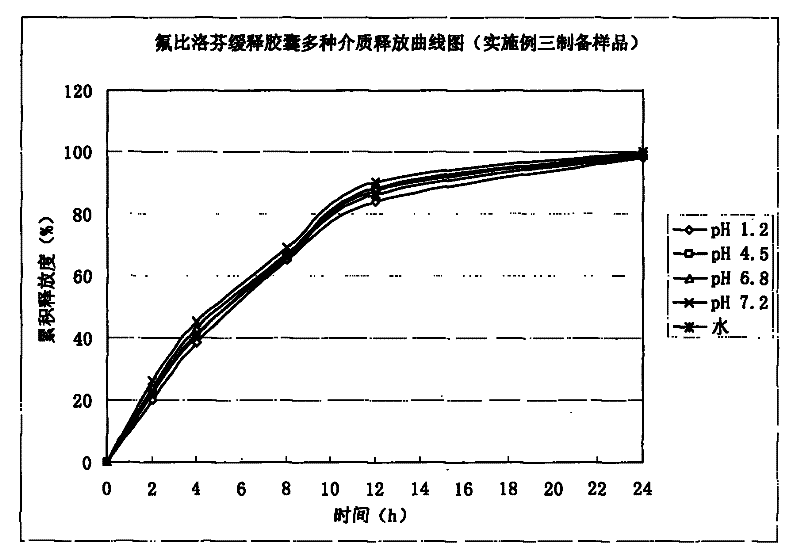
Embodiment 3
[0058] 1. Preparation of drug-containing pill core:
[0059]
[0060] Preparation process: Mix flurbiprofen, microcrystalline cellulose, and mannitol uniformly by increasing the method in equal amounts, add PVP-K30 and Tween to 35% ethanol solution, make the above mixed powder into a soft material, and extrude The spheronization mechanism pellets are produced, the particle size of the sieve plate is 0.8mm, oven-dried at 60°C, sieved, and 20-40 mesh-containing pellet cores are selected for coating.
[0061] 2. Package slow-release layer:
[0062]
[0063] Add the prescribed amount of talc powder into purified water and stir for 45 minutes, add triethyl citrate, Eudragit RS30D and RL30D water dispersion to make it fully dispersed, continue stirring for 45 minutes, and set aside. The pill core containing the drug is placed in a fluidized bed, and various process parameters are adjusted to ensure a fluidized state, and the weight of the coating is increased to 8%.
[0064]...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com