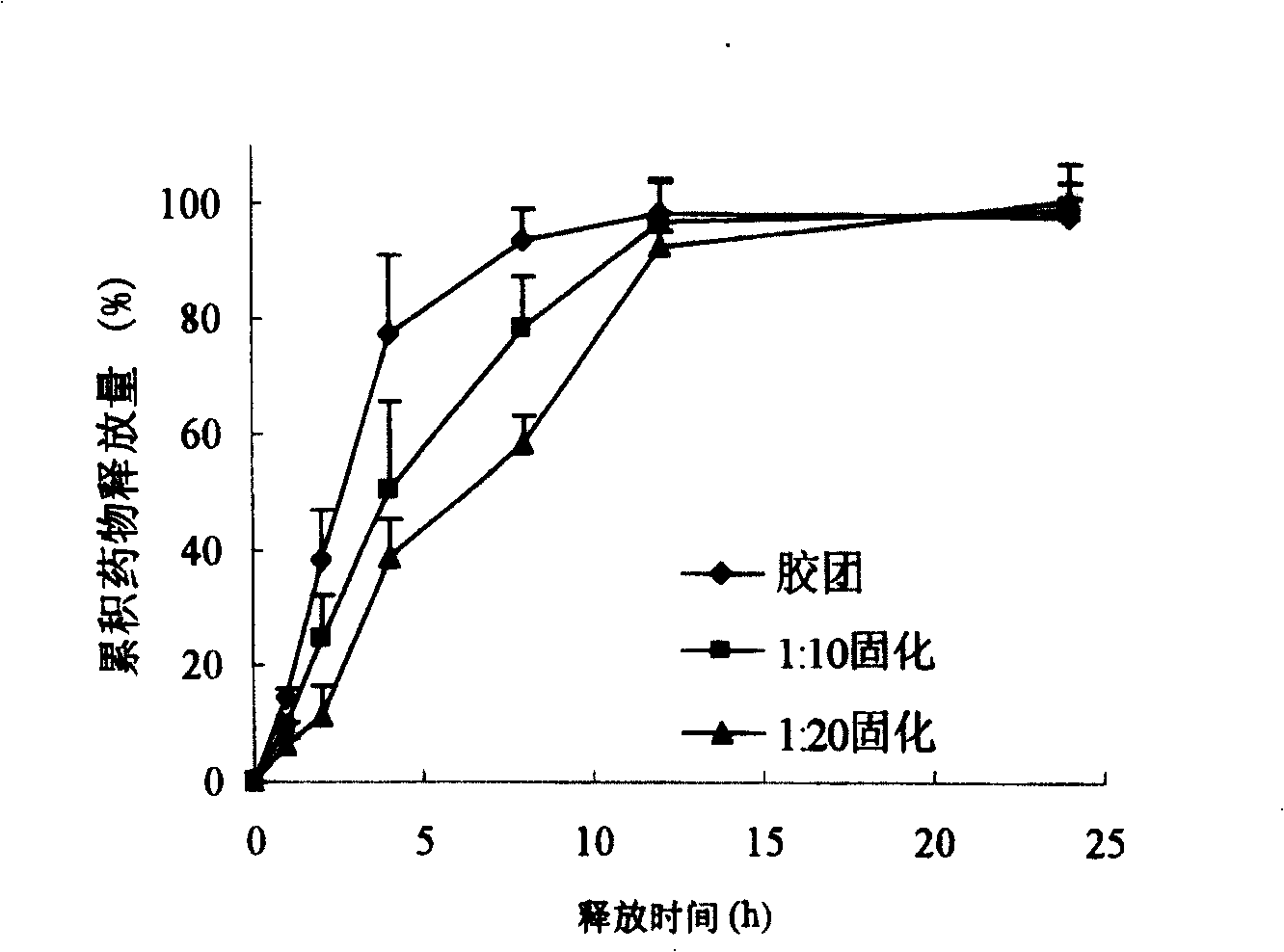
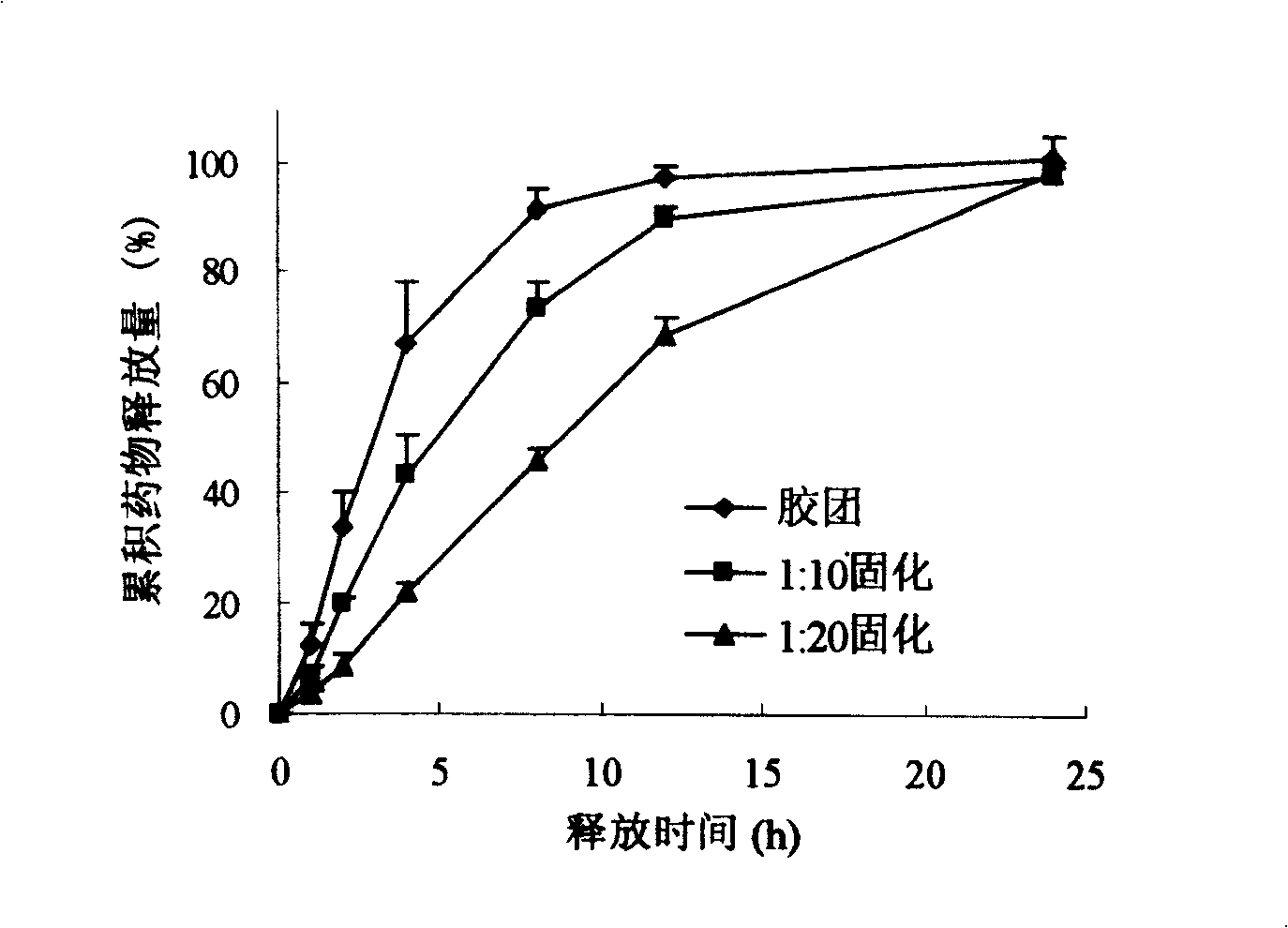
Surface-modified hydrophobically modified drug-carried chitosan polymer micelle and method for preparing same
A hydrophobic modification and surface modification technology, applied in the field of surface modification of polymer micelles, to achieve the effects of improving instability, improving curative effect and reducing drug burst release
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] 1. Preparation of chitosan oligosaccharide
[0037] Add 6g of chitosan (average molecular weight 450kDa) to 200mL of 1.25% (v / v) hydrochloric acid aqueous solution, stir and dissolve at 55-60°C, adjust the pH to 5.0 with dilute ammonia water or dilute hydrochloric acid, press cellulase and shell The ratio of polysaccharides is 0.5:100 (w / w) to add cellulase, and after controlling the reaction time for 8 hours, the reaction product is 4000r×min -1 After centrifugation for 10 minutes, the supernatant was pretreated with a 0.45 μm microporous membrane, graded by ultrafiltration with ultrafiltration membranes of different molecular weights, and the ultrafiltrate was freeze-dried to obtain chitosan oligosaccharides with a certain molecular weight. And the average molecular weight was determined to be 50.6 kDa by gel permeation chromatography.
[0038]2. Preparation of hydrophobically modified chitosan oligosaccharides
[0039] Get above-mentioned chitosan oligosaccharide 5...
Embodiment 2
[0050] 1. Preparation of chitosan oligosaccharide
[0051] Add 6g of chitosan (average molecular weight 450kDa) to 200mL of 1.25% (v / v) hydrochloric acid aqueous solution, stir and dissolve at 55-60°C, adjust the pH to 5.0 with dilute ammonia water or dilute hydrochloric acid, press cellulase and shell The ratio of polysaccharides is 0.5:100 (w / w) to add cellulase, and after controlling the reaction time for 8 hours, the reaction product is 4000r×min -1 After centrifugation for 10 minutes, the supernatant was pretreated with a 0.45 μm microporous membrane, graded by ultrafiltration with ultrafiltration membranes of different molecular weights, and the ultrafiltrate was freeze-dried to obtain chitosan oligosaccharides with a certain molecular weight. And the average molecular weight was determined to be 50.6 kDa by gel permeation chromatography.
[0052] 2. Preparation of hydrophobically modified chitosan oligosaccharides
[0053] Get above-mentioned chitosan oligosaccharide ...
Embodiment 3
[0064] 1. Preparation of chitosan oligosaccharide
[0065] Add 6g of chitosan (average molecular weight 450kDa) to 200mL of 1.25% (v / v) hydrochloric acid aqueous solution, stir and dissolve at 55-60°C, adjust the pH to 5.0 with dilute ammonia water or dilute hydrochloric acid, and mix with cellulase Chitosan ratio 0.5:100 (w / w) was added to cellulase, and after controlling the reaction time for 8 hours, the reaction product was 4000r×min -1 After centrifugation for 10 minutes, the supernatant was pretreated with a 0.45 μm microporous membrane, graded by ultrafiltration with ultrafiltration membranes of different molecular weights, and the ultrafiltrate was freeze-dried to obtain chitosan oligosaccharides with a certain molecular weight. And the average molecular weight was determined to be 50.6 kDa by gel permeation chromatography.
[0066] 2. Preparation of hydrophobically modified chitosan oligosaccharides
[0067] Get above-mentioned chitosan oligosaccharide 5.0g, accurat...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com