Synthetic peptide vaccine for O-type foot and mouth disease of swine and preparation method thereof
A technique for synthesizing peptide vaccines and foot-and-mouth disease, applied in the field of genetic engineering, can solve problems such as high cost and complex production process, and achieve the effects of low production cost, simple separation and purification, and high expression efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
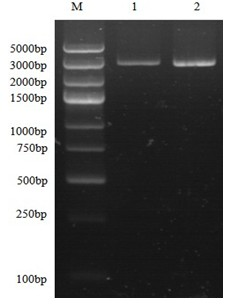
[0025] (1) Construction of recombinant expression vector: According to the partial tropism of the Pichia pastoris gene, the amino acid sequence of the synthetic peptide SP, and the multiple cloning site of the expression vector pGAPZαA, the SP gene was designed and synthesized, connected to the plasmid Puc57 vector, and double-enzyme Cut the Puc57 recombinant vector and the expression vector pGAPZαA, and then connect and transform to construct the recombinant expression vector ( figure 1 ). Another two primers were designed for detection, the primers are as follows: p1: SEQ ID NO: 4, p2: SEQ ID NO: 5, and the enzyme cutting sites are EcoRI and NotI.
[0026] (2) Construction of genetically engineered bacteria: Transform the host bacteria with the recombinant expression vector constructed above, and the host bacteria is Pichia pastoris X33.
[0027] (3) Expression of peptides synthesized by recombinant yeast: Use a sterilized toothpick to pick out a single colony with Zeocin ...
Embodiment 2
[0030] Preparation of pig O-type foot-and-mouth disease synthetic peptide vaccine: SDS-PAGE and Western-Blot analysis of the supernatant expressed in Figure 3~4 ), and then mixed penicillin and streptomycin with the separated and purified protein, and adjusted the pH value of the mixture to the physiological value, that is, pH7. agent.
Embodiment 3
[0032] Determination of expression level of swine type O foot-and-mouth disease synthetic peptides: Bradford protein concentration assay kit was used for the determination of SP expression supernatant protein concentration. Since there is a small amount of bacterial protein in the expression supernatant, the measured concentration is the total protein concentration. The specific steps are as follows:
[0033]1. Completely dissolve protein standard BSA (5 mg / mL), take 10 μL and dilute to 100 μL, so that the final concentration is 0.5 mg / mL. Standards were diluted with PBS.
[0034] 2. Add 0, 1, 2, 4, 8, 12, 16, 20 μL of the diluted standard to the standard wells of the 96-well plate, and add PBS diluent to make up to 20 μL.
[0035] 3. Add 10 μL of the supernatant of synthetic peptides expressed at 48h, 72h, and 96h to the sample wells of the 96-well plate, and add PBS diluent to 20 μL.
[0036] 4. Add 200 μL of G250 staining solution to each well and let stand at room tempera...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com