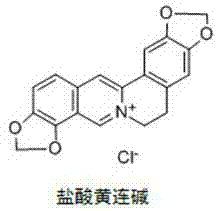
Application of coptisine serving as indoleamine 2, 3-dioxygenase (IDO)inhibitor
A technology of dioxygenase and coptisine, applied in the field of medicine, can solve problems such as unreported, and achieve the effect of good potential of research and development of new drugs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] 1. Preliminary detection of IDO inhibitory activity
[0022] 19(1): 22-9)。 The construction of the plasmid containing the human IDO gene, expression in E. coli, extraction and purification were carried out according to the method reported by Littlejohn et al. (Protein Exp Purif, 2000. 19(1): 22-9). The inhibitory activity of compounds against IDO was tested as described. Mix 50 mM potassium phosphate buffer (pH 6.5), 40 mM vitamin C, 400 μg / ml catalase, 20 μM methylene blue, substrate L-tryptophan and the sample to be tested, and incubate the mixture at 37 °C for 5 minutes , then IDO enzyme was added to the above mixture, the reaction was carried out at 37 °C for 30 minutes, 200 μl of 30% (w / v) trichloroacetic acid was added to terminate the reaction, and the reaction system was heated at 65 °C for 15 minutes to complete the reaction. Conversion of formylkynurenine to kynurenine, followed by centrifugation at 12,000 rpm for 10 minutes, the supernatant was mixed with an...
Embodiment 2
[0037] Example 2: Response to β-amyloid (Aβ 1-42 ) and γ-interferon (IFN-γ) combined-induced neuronal cell injury and protection against apoptosis
[0038] 1. Method
[0039] 1.1. Cell Lines and Cell Culture
[0040] The rat neuronal cell line PC12 was used. PC12 cells were cultured in DMEM medium with 10% inactivated fetal bovine serum, 50U / ml penicillin, and 25μg / ml streptomycin. Cells were placed at 37 °C with 5 % CO 2 In the incubator, passage 1:4 when 80%-90% confluent.
[0041] 1.2. Establishment of Alzheimer's disease (AD) cell model
[0042] When PC12 cells grow to 60% confluence in a 6-well plate, add 1 ml of 25 μM Aβ 1-42 DMEM medium for 24 h. Then change to 1ml of 1×10 3 The AD neuron model was established after the DMEM medium of U / mL IFN-γ was cultured for 24 h.
[0043] 1.3. Grouping
[0044] The cells in this experiment were divided into 5 groups, namely blank group, Aβ group, Aβ+IFN-γ group, coptisine group, and 1-MT positive control group. Blank gro...
Embodiment 3
[0067] Example 3: Influence on the learning and memory ability of Alzheimer's rats
[0068] 1. Experimental method:
[0069] 1.1 Main reagents and instruments
[0070] L-kynurenine (L-kynurenine, L-KYN), L-tryptophan (L-Tryptophan, L-TRP) standards were purchased from sigma, the product numbers are K8625, T8941, respectively. Morris Water Maze: Shanghai Shift Information Technology Co., Ltd. High performance liquid chromatograph: Shimadzu LC-10A, Japan.
[0071] 1. 2. Experimental animals
[0072] APP-PS1 Alzheimer's disease mice (AD mice), 40 males and 12 wild-type B6C3 mice (WT mice), weighing 35±5 g, were purchased from Nanjing Animal Research Institute. APP-PS1 double-transgenic mice can express the fusion of mutant human presenilin (DeltaE9) and human-mouse amyloid preprotein (APPswe), and can reproduce and survive normally. Usually there are more senile plaques in the brain after 30 weeks of age, showing symptoms of dementia, and it is used as a model organism for A...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com