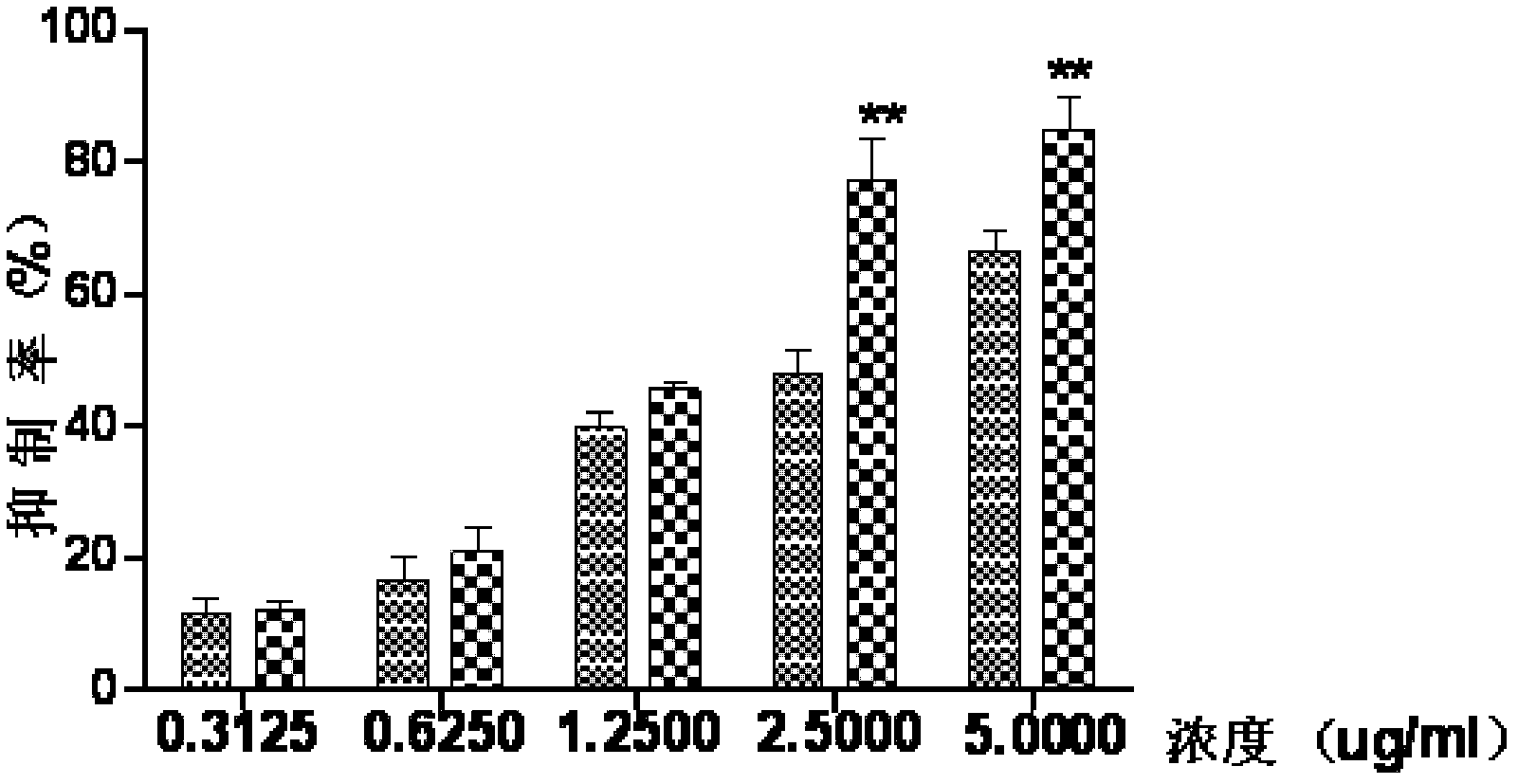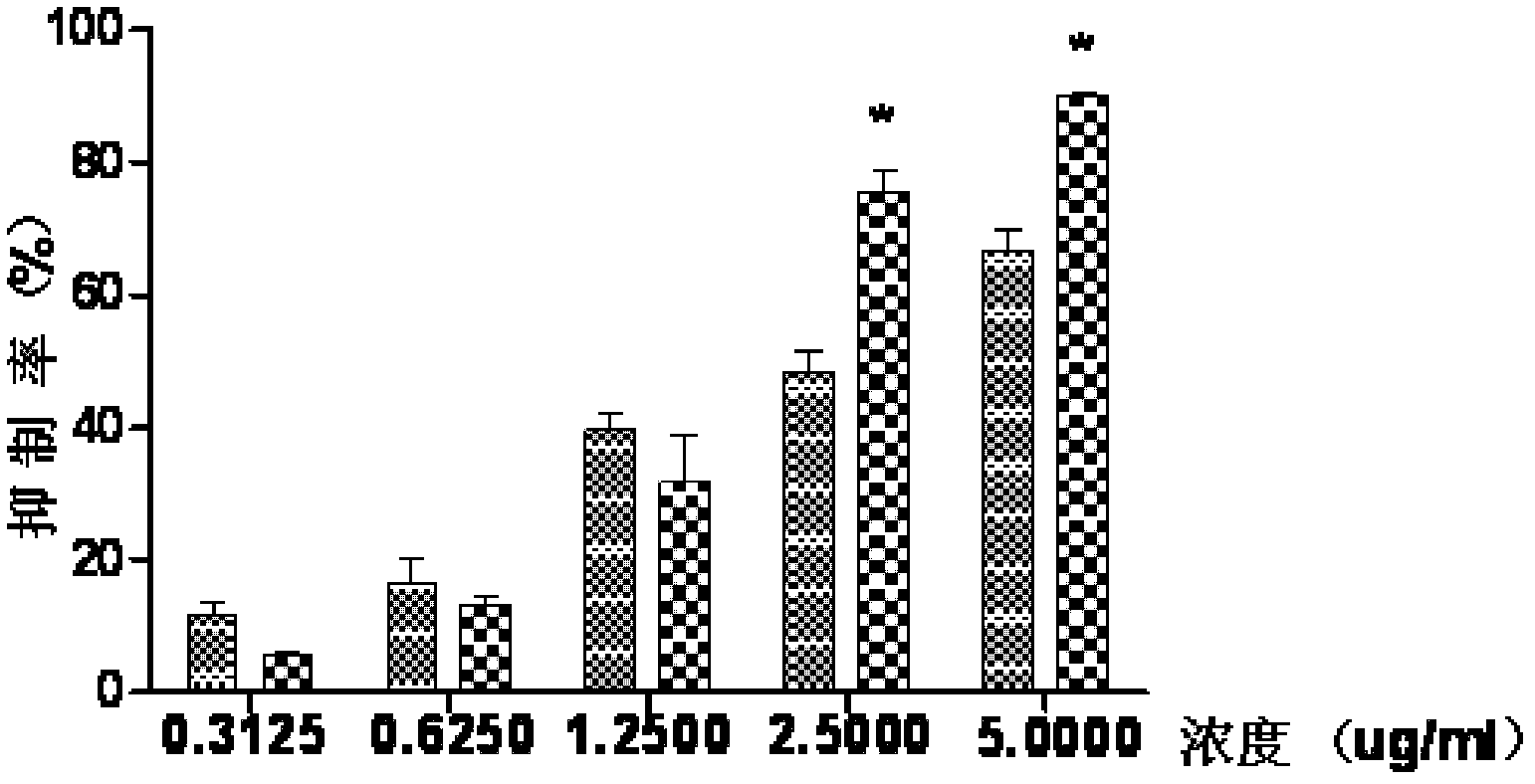Medicament composition containing ginsenoside and cantharidin and application of medicament composition
A technology of ginsenosides and total ginsenosides is applied in the directions of drug combinations, medical preparations containing active ingredients, active ingredients of heterocyclic compounds, etc., which can solve problems such as clinical application limitations, reduce the incidence of adverse reactions, and improve safety. , Improve the effect of anti-tumor effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Embodiment 1: Preparation of total ginsenosides
[0045] 20kg ginseng, cut into small pieces of 1-2cm, add 160L 50% ethanol to reflux and extract twice, the first time for 3 hours, the second time for 1.5 hours, combine the extracts, centrifuge at 4500rpm for 20 minutes, take the supernatant and filter it with filter paper, collect The filtrate is used for later use.
[0046] Get 5kg of processed AB-8 resin, put it into a stainless steel chromatographic column with a diameter of 30cm, the diameter-to-height ratio of the resin bed is 1:10, wash with water, and filter the obtained ginseng extract at a rate of 1mL / min.cm 2 The sample was loaded twice at a high speed, and the static adsorption was performed for 12 hours. First wash 4BV with water and discard; then wash 3BV with 30% ethanol and discard; finally use 75% ethanol at 2-3mL / min.cm 2 Speed elution, collect the eluate, concentrate and dry to obtain 50g of total ginsenosides.
Embodiment 2
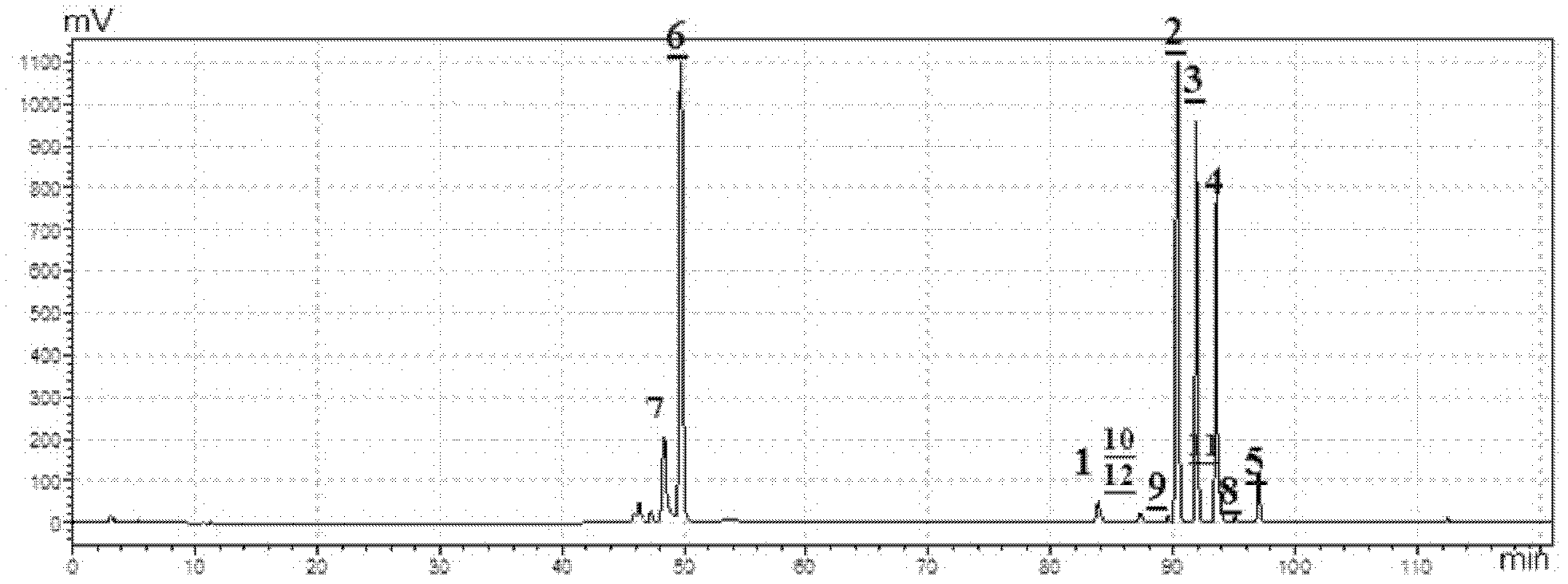
[0047] Embodiment 2: The HPLC-ELSD pattern determination of total ginseng saponins
[0048] The total ginsenosides prepared in Example 1 were taken for HPLC-ELSD spectrum determination. Chromatographic column: Kromasil C18 (250mm×4.6mm, 5.0μm). Mobile phase: A is acetonitrile, B is water, gradient elution program: 0~10min, 5%A~20%A; 10~35min, 20%A; 10~35min, 20%A~29%A; 36~ 70min, 29%A; 70~100min, 47%A. Flow rate: 1mL·min-1; Column temperature: 35°C. Injection volume: 20 μL. Evaporative light scattering detector: T=40°C, P=0.35MPa, the carrier gas is high-purity nitrogen. Test results such as figure 1 shown.
[0049] Depend on figure 1 Visible, the total ginsenosides that the embodiment of the present invention 1 makes comprises ginsenoside Rf, Rb 1 , Rc, Rb 2 , Rd, Rg 1 , Re, 3-O-β-D-glucopyranosyl-oleanolic acid-28-O-β-D-glucopyranoside, notoginsenoside NR 4 , 3-O-α-L-arabinofuranosyl (2-1)-α-L-rhamnopyranose (3-1)-β-D-glucopyranose-(4-1)-β-D -Glucopyranosyl--Olea...
Embodiment 3
[0052]Embodiment 3: the preparation of mylabris extract
[0053] Weigh 20g of mylabris powder, pour it into a 1000mL round bottom flask, add 250mL of acetone, shake intermittently at 30°C for 2h, and extract twice. The extracts were combined, concentrated under reduced pressure, washed with 95% ethanol:petroleum ether (1:1.5) until the washing liquid was colorless, and a near-white crude product was obtained, then about 5mL of acetone was added, heated to dissolve, filtered while hot, and the filtrate was placed at 4°C Natural crystallization gave 50 mg of white transparent needle crystals, yield 0.25%. The product was detected by liquid chromatography and gas chromatography, and no impurity peaks were found, indicating that the product had a purity greater than 99.5%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com