Preparation method for LiNi0.5Mn1.5O4
A compound and nickel source technology, applied in electrical components, battery electrodes, circuits, etc., can solve the problems of low purity, low specific capacity of lithium-ion batteries, and reduced cycle life of lithium-ion batteries, and achieve low oxygen defects and high purity. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0030] The invention discloses a LiNi 0.5 Mn 1.5 O 4 The preparation method includes:
[0031] Step a) mixing the nickel source compound and the manganese source compound in a solvent and drying;
[0032] Step b) calcining the dried mixture in step a) at 900-1000°C to obtain a nickel manganese oxide precursor;
[0033] Step c) mixing the nickel manganese oxide precursor with a lithium source compound, and sintering at 700-750°C;
[0034] Step d) Annealing the sintered product in step c in an oxygen atmosphere to obtain LiNi 0.5 Mn 1.5 O 4 .
[0035] According to the present invention, the solvent in the step a) is preferably deionized water, and the nickel source compound and manganese source compound are mixed in the solvent, and preferably formulated to have a mass concentration of 0.4 mol / L to 0.7 mol / L The mass concentration of the solution is more preferably 0.5 mol / L. The drying method in the step a) is preferably a spray drying method, a freeze drying method, and the like. The...
Embodiment 1
[0042] Weigh nickel acetate and manganese acetate with a molar ratio of Ni:Mn of 0.5:1.5, mix them and add deionized water to prepare a 0.5mol / L solution, and dry the resulting solution with a spray dryer to obtain a mixed powder;
[0043] The obtained powder is calcined in an air atmosphere at a constant temperature of 900°C for 20 hours;
[0044] After natural cooling, add lithium acetate with a molar ratio of 2:1 to Ni, grind and mix uniformly, and then calcinate in an air atmosphere at 700°C for 24 hours to obtain untreated LiNi 0.5 Mn 1.5 O 4 ;
[0045] The untreated LiNi 0.5 Mn 1.5 O 4 Calcined in an oxygen atmosphere at a constant temperature of 500°C for 30 hours and cooled to room temperature in air to obtain LiNi 0.5 Mn 1.5 O 4 .
Embodiment 2
[0047] Weigh nickel acetate and manganese acetate with a molar ratio of Ni:Mn of 0.5:1.5, mix them and add deionized water to prepare a 0.5mol / L solution, and dry the resulting solution with a spray dryer to obtain a mixed powder;
[0048] Calcining the obtained powder in an air atmosphere at a constant temperature of 900°C for 20 hours;
[0049] After natural cooling, add lithium hydroxide with a molar ratio of 2:1 to Ni, grind and mix uniformly, and then calcinate in an air atmosphere at 700°C for 24 hours to obtain untreated LiNi 0.5 Mn 1.5 O 4 ;
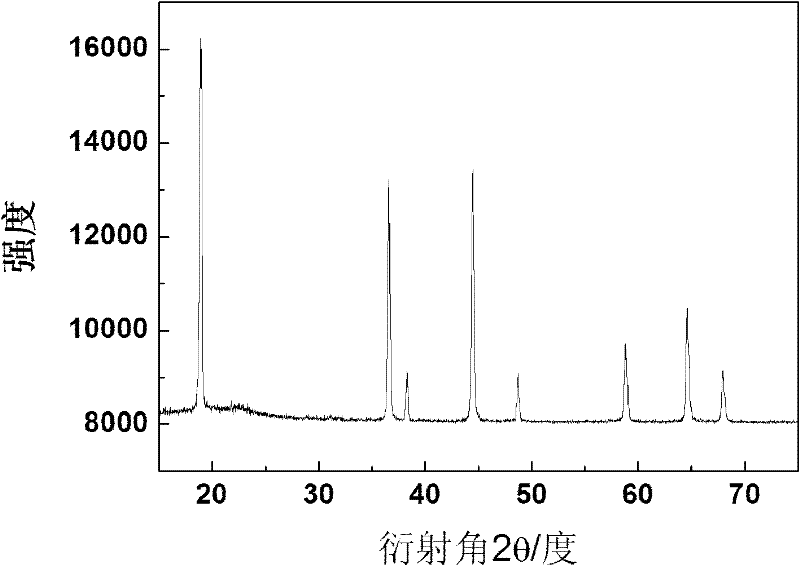
[0050] The untreated LiNi 0.5 Mn 1.5 O 4 Calcined in an oxygen atmosphere at a constant temperature of 500°C for 30 hours, and cooled to room temperature in an oxygen atmosphere to obtain LiNi 0.5 Mn 1.5 O 4 . Such as figure 1 As shown, the LiNi prepared in this example 0.5 Mn 1.5 O 4 It can be seen from the figure that there is no diffraction peak of impurity phases such as NiO. Therefore, the LiNi prepared in this example 0.5 Mn 1.5 O...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com