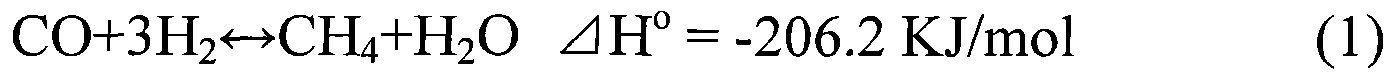Methanation catalyst and preparation method thereof
A methanation catalyst and catalyst technology, applied in the direction of physical/chemical process catalysts, chemical instruments and methods, metal/metal oxide/metal hydroxide catalysts, etc., can solve the problem of narrow operating temperature range of methane catalysts, and achieve improved Better performance, improve the efficiency of metal use, and facilitate the effect of diffusion
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] Weigh the alumina (γ-Al 2 o 3 ) 100 grams of carrier, denoted as A.
[0049] 6.68 grams of magnesium nitrate [Mg(NO 3 ) 2 ·6H 2 O] Dissolved with 85 grams of deionized water, dipped on A, baked at 110° C. for 3 hours, and then roasted and decomposed at 350° C. and 450° C. for 2 hours respectively in an air atmosphere to obtain B1.
[0050] 109.7 grams of nickel nitrate [Ni(NO 3 ) 2 ·6H 2 O], 3.6 grams of 50% manganese nitrate [Mn(NO 3 ) 2 ] with 27.1 grams of citric acid [C 6 h 8 o 7 ·H 2 O] miscible, the ratio of the total moles of carboxyl groups to the total moles of metal ions is 0.5, after dissolving with 51 grams of deionized water, impregnate on B1. Bake at 110°C for 5 hours to obtain C1.
[0051] After C1 was decomposed by roasting at 300°C and 450°C for 3 hours in an air atmosphere, a catalyst containing 20% by weight of nickel, 0.5% by weight of manganese and 0.5% by weight of magnesium was obtained, and the components nickel and manganese only...
Embodiment 2
[0054] Weigh the alumina (γ-Al 2 o 3 ) 100 grams of carrier, denoted as A.
[0055] 169.3 grams of nickel nitrate [Ni(NO 3 ) 2 ·6H 2 O] with 81.6 grams of citric acid [C 6 h 8 o 7 ·H 2 O] miscibility, the ratio of the total moles of carboxyl groups to the total moles of nickel ions is 1, after adding 82 grams of deionized water to dissolve, impregnate on A. Baked at 110°C for 5 hours, and then fired at 300°C and 400°C for 2 hours to decompose to obtain B2.
[0056] 89.0 grams of 50% manganese nitrate [Mn(NO 3 ) 2 ], 34.8 g citric acid [C 6 h 8 o 7 ·H 2 O], the ratio of the total moles of carboxyl groups to the total moles of manganese ions is 1, after adding 12 grams of deionized water to dissolve, impregnate on B2. Bake at 110°C for 4 hours, and then bake at 300°C and 350°C for 2 hours to decompose to obtain C2.
[0057] 4.9 grams of lanthanum nitrate [La(NO 3 ) 3 ·6H 2 O] after dissolving with 89 grams of deionized water, impregnate on C2, bake 2 hours at ...
Embodiment 3
[0060] Weigh the alumina (γ-Al 2 o 3 ) 100 grams of carrier, denoted as A3.
[0061] 6.68 grams of magnesium nitrate [Mg(NO 3 ) 2 ·6H 2 O] After dissolving with 85 grams of deionized water, impregnate A3, bake at 110°C for 3 hours, and then roast and decompose it at 300°C and 450°C for 2 hours in an air atmosphere to obtain B3.
[0062] 109.7 grams of nickel nitrate [Ni(NO 3 ) 2 ·6H 2 O], 3.6 grams of 50% manganese nitrate [Mn(NO 3 ) 2 ] with 27.1 grams of citric acid [C 6 h 8 o 7 ·H 2 O] miscible, the ratio of the total moles of carboxyl groups to the total moles of metal ions is 0.5, after adding 50 grams of deionized water to dissolve, impregnate on B3. Bake at 110°C for 5 hours to obtain C3.
[0063] After C3 is roasted and decomposed at 300°C and 450°C for 4 hours in an air atmosphere, a catalyst containing 20% by weight of nickel, 0.5% by weight of manganese and 0.5% by weight of magnesium is obtained, and the components nickel and manganese only exist in...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com