Method for cladding LiNi0.133Co0.133Mn0.544O2 material
A technology of coating and lithium salt, which is applied in the field of high specific energy lithium-rich cathode materials for lithium-ion batteries, to achieve the effect of improving coating effect, improving electrochemical performance and preventing contact
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Add 0.4224g of lithium acetate to 200ml of ethanol, then add 10g of citric acid, stir to dissolve, then add 1.3176g of pentaethoxyniobium to the solution, stir and mix well, then add 30g of LiNi 0.133 co 0.133 mn 0.544 o 2 Stir for 12 hours to make them fully mix to form a sol; then heat and stir at 80°C to slowly volatilize the ethanol solution to form a dry gel precursor; grind the precursor evenly and put it in a muffle furnace to The temperature was raised to 800°C at a rate of 5°C / min, and the constant temperature was calcined for 5 hours in an air atmosphere; during this high-temperature calcination process, LiNi 0.133 co0.133 mn 0.544 o 2 LiNiO formed on the surface of the material 3 crystals, obtained after natural cooling coated with LiNiO 3 LiNi 0.133 co 0.133 mn 0.544 o 2 Cathode material.
Embodiment 2
[0030] Add 0.1530g of lithium carbonate to 200ml of ethanol, then add 10g of citric acid, stir to dissolve, then add 1.3176g of pentaethoxyniobium into the solution, stir and mix well, then add 30g of LiNi 0.133 co 0.133 mn 0.544 o 2 Stir for 12 hours to make them fully mix to form a sol; then heat and stir at 80°C to slowly volatilize the ethanol solution to form a dry gel precursor; grind the precursor evenly and put it in a muffle furnace to The temperature was raised to 600°C at a rate of 5°C / min, and the constant temperature was calcined for 4 hours in an air atmosphere; during this high-temperature calcination process, LiNi 0.133 co 0.133 mn 0.544 o 2 LiNiO formed on the surface of the material 3 crystals, obtained after natural cooling coated with LiNiO 3 LiNi 0.133 co 0.133 mn 0.544 o 2 Cathode material.
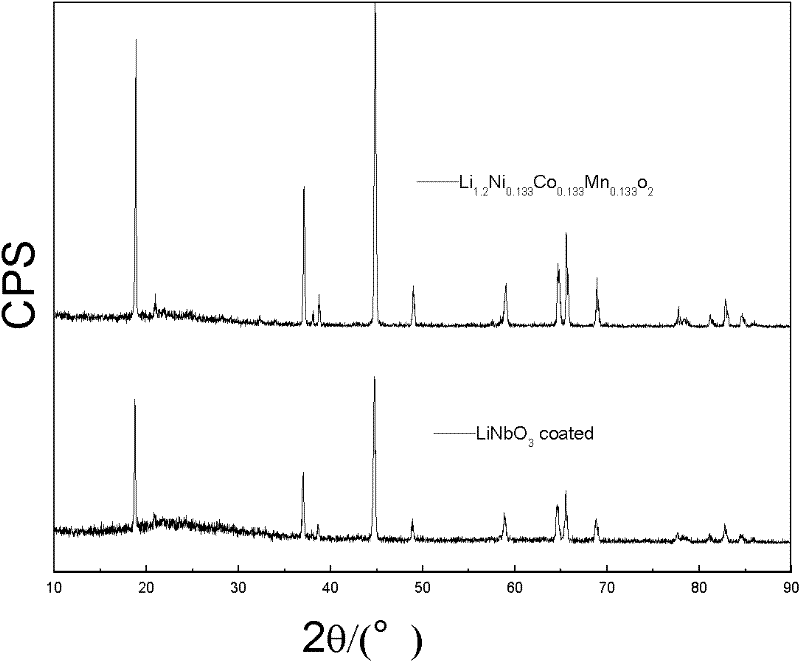
[0031] according to figure 1 The test results show that the coated LiNiO 3 LiNi before and after 0.133 co 0.133 mn 0.544 o 2 material, its XRD patt...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com