Docetaxel pH-sensitive liposome and preparation method thereof
A technology of docetaxel and liposome, applied in the field of pH-sensitive liposome and its preparation, can solve problems such as large toxic and side effects, and achieve the effects of low toxic and side effects, high drug therapeutic index and good storage stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
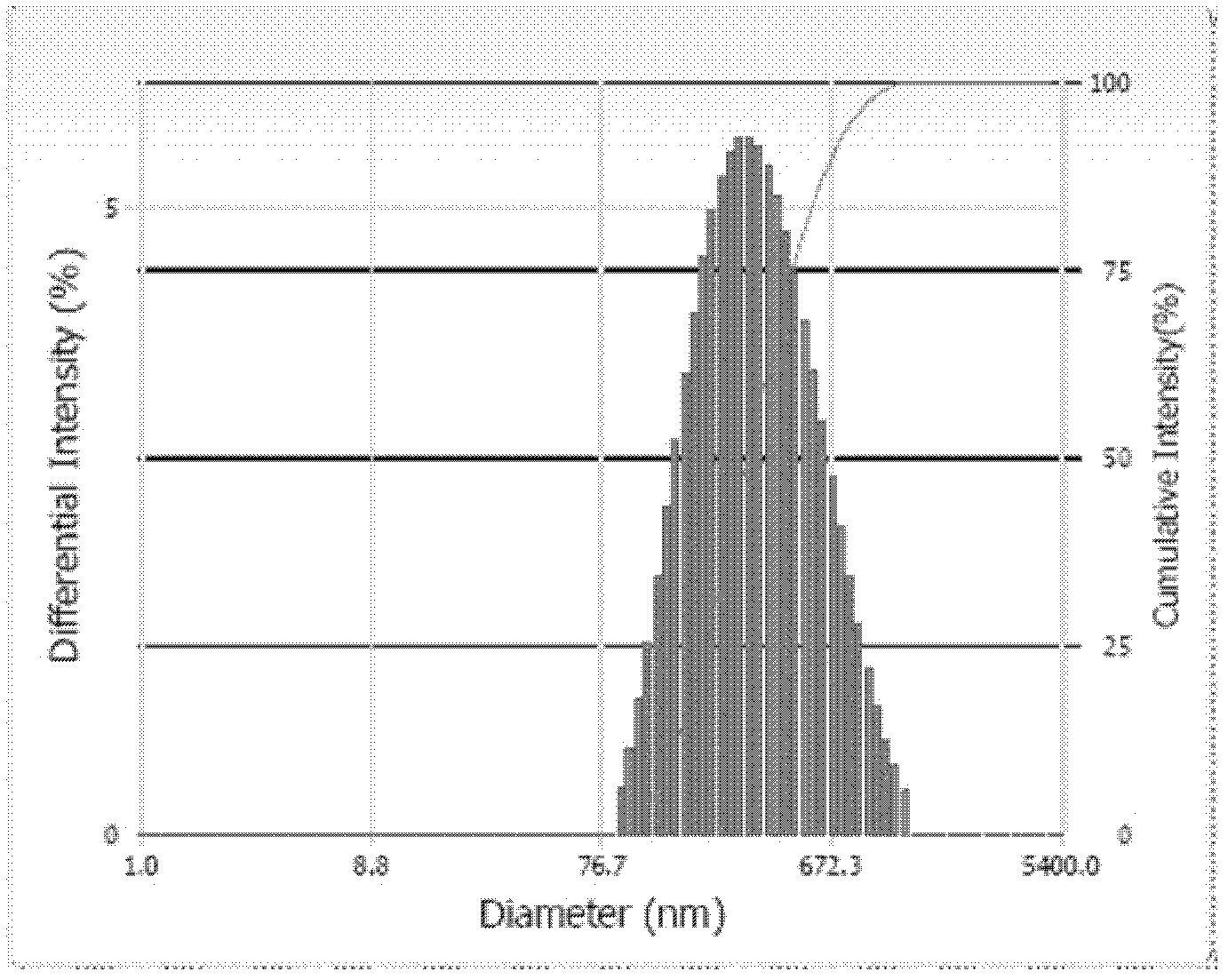
[0019] Weigh 30 mg of phosphatidylethanolamine, 20 mg of cholesterol, and 30 mg of oleic acid, dissolve them in 15 ml of organic solvent chloroform, and evaporate the mixed solution to dryness under pressure until a uniform lipid film is formed. Add 2ml of docetaxel drug solution (drug concentration: 5mg / ml) with pH=7.4 to dissolve, sonicate to form a uniform suspension, supplement with PBS buffer with pH7.4 to make the final volume 20ml, and hydrate in a water bath at 37°C After 30 minutes, the docetaxel pH-sensitive liposome was ready. The prepared docetaxel pH-sensitive liposome particle size is 277 ± 2nm~303 ± 1nm, and the average particle size is 286.4nm. figure 1 As shown, the particle size distribution is as figure 2 shown.
Embodiment 2
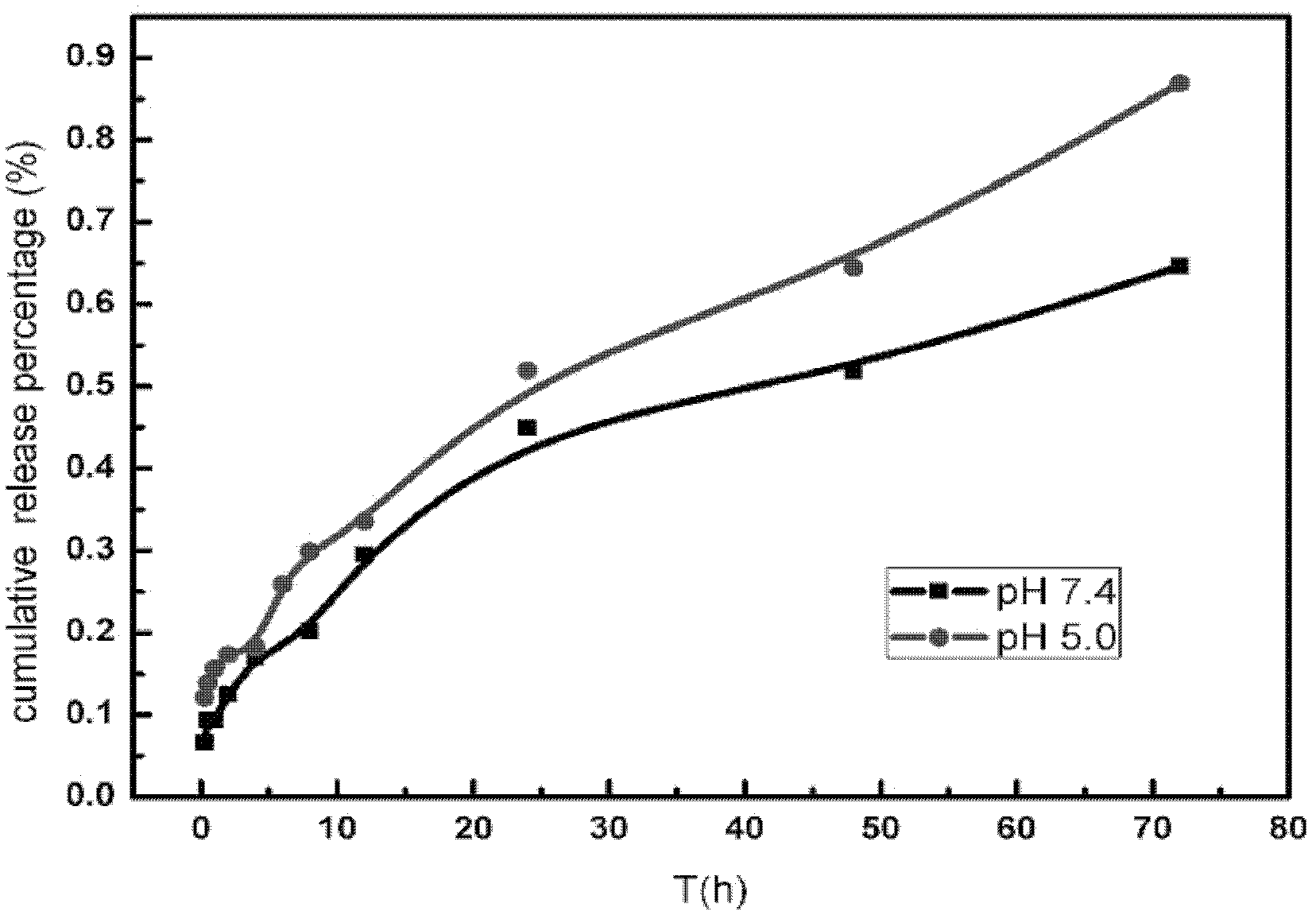
[0021] Docetaxel pH-sensitive liposomes were prepared according to Example 1 above. Utilize the dynamic membrane dialysis method, investigate the release law of preparation in different pHPBS, the obtained release curve is as follows: image 3 As shown, it can be seen that the release rate of the liposome-encapsulated drug is different in different pH environments, and the release rate of the drug is faster in an acidic environment.
Embodiment 3
[0023] Using the docetaxel pH-sensitive liposomes prepared in the above-mentioned Example 1 and the control solution formulated with the docetaxel bulk drug, dynamic membrane dialysis was used to investigate their release in the same pH buffer solution. Law as Figure 4 As shown, it can be seen that preparing the drug into liposomes can delay the release of the drug and improve its bioavailability.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com