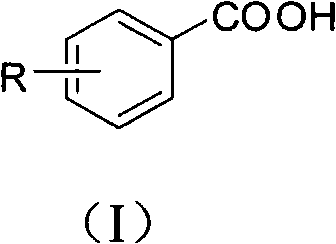
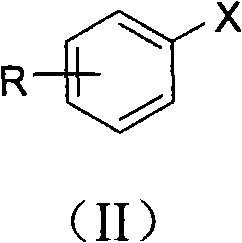
Preparation method for aromatic carboxylic acid compounds
A technology for aromatic carboxylic acids and compounds, which is applied in the field of organic synthesis and can solve the problems of expensive catalysts and ligands
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] Embodiment 1, the preparation of p-toluic acid
[0049] Add cuprous iodide (0.2mmol, 38mg), L-proline (0.2mmol, 23mg), Cs 2 CO 3 (2mmol, 652mg), p-methyliodobenzene (1mmol, 218mg), malononitrile (2mmol, 132mg) and solvent dimethyl sulfoxide (1mL), the pH value of the reaction system is 10, at 140 ° C, in the air In the presence of conditions, react for 48 hours; after the reaction is completed, cool to room temperature, remove the solvent dimethyl sulfoxide with a rotary evaporator, add 2 mL of 1M hydrochloric acid (pH = 2 ~ 3) for acidification, and then use ethyl acetate The ester was extracted 3 times, 2 mL each time, the combined organic phase was concentrated, and purified with a silica gel column (the specification of silica gel was 200-300 mesh, and the eluent was petroleum ether / ethyl acetate (3:1, v / v )) to obtain p-toluic acid 75mg, the productive rate was 55%.
[0050] Product p-toluic acid: 1 H NMR (CDCl 3 , 600MHz, ppm) δ12.59(s, br, 1H), 7.93(d, 2H, J...
Embodiment 2
[0051] Embodiment 2, the preparation of p-toluic acid
[0052] Proceed according to the steps described in Example 1, but replace cesium carbonate with potassium carbonate as the base, and the yield of p-toluic acid is 49%.
Embodiment 3
[0053] Embodiment 3, the preparation of p-toluic acid
[0054] Carry out according to the procedure described in Example 1, but replace cesium carbonate with potassium phosphate as the base, and the yield of p-toluic acid is 45%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com