Medicament carrying nano particles and preparation method and application thereof
A nanoparticle and drug technology, which is applied in the direction of drug combination, pharmaceutical formula, antineoplastic drugs, etc., can solve the problems of filter clogging, patient drug administration impact, nanoparticle precipitation reduces drug stability and drug efficacy, etc., and achieves good water dispersion sexual effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
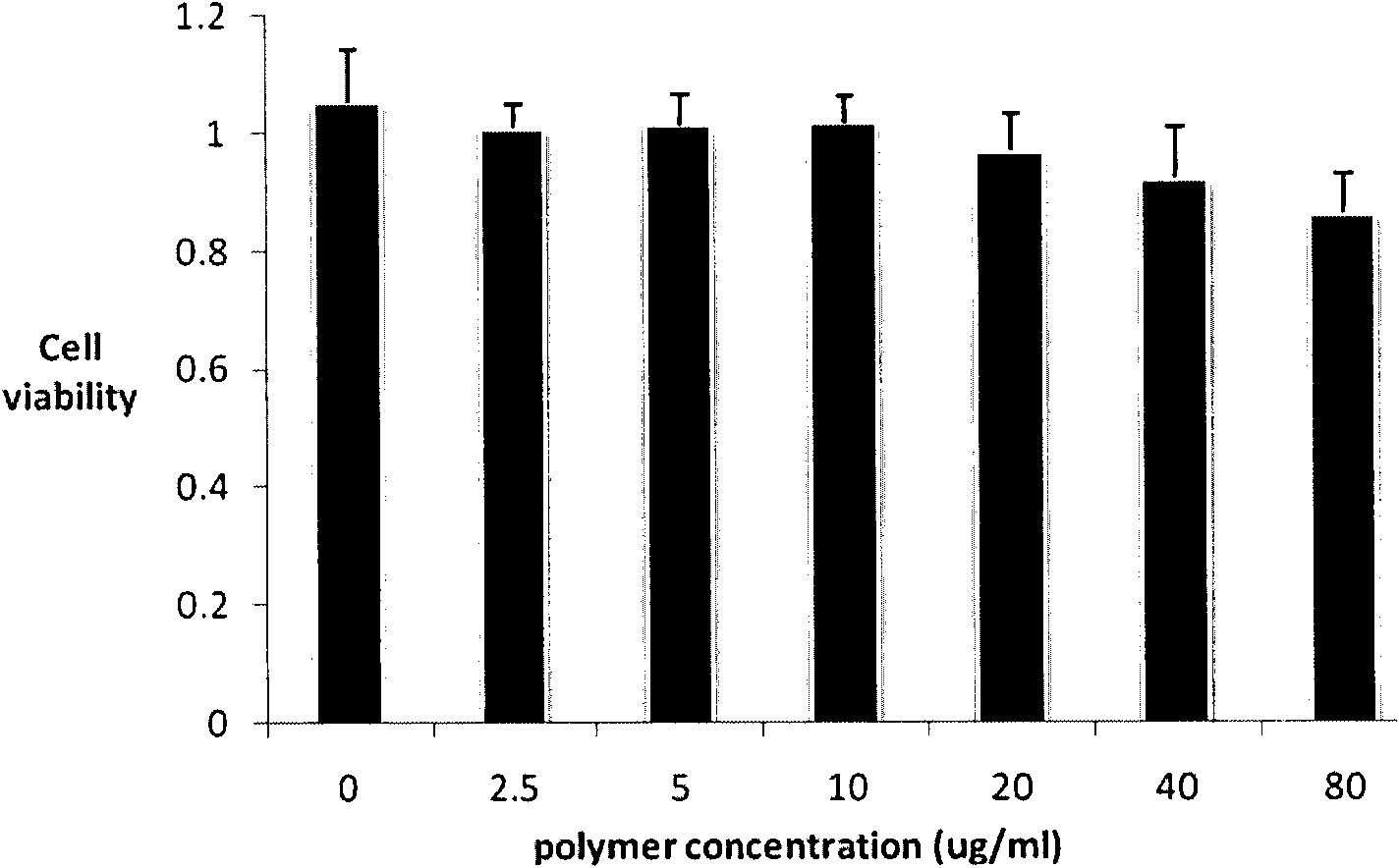
Method used
Image
Examples
Embodiment 1
[0050] Embodiment 1: Preparation of polylactic acid-polyethylene glycol diblock copolymer blank nanoparticles
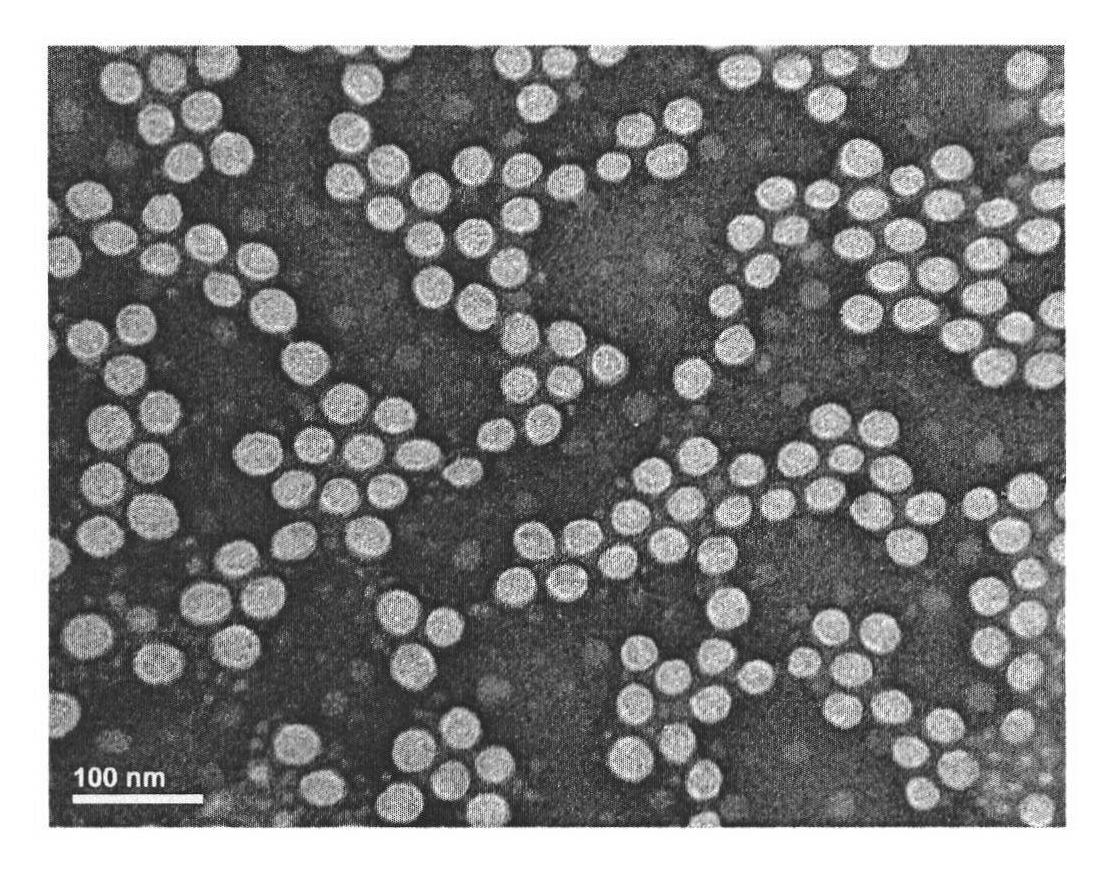
[0051] Accurately weigh 20mg of PLGA-mPEG into a 5mL tube, dissolve in 2mL of acetone, and slowly drop the acetone solution of the polymer into the aqueous solution at room temperature under a magnetic stirrer (1 drop / 10s, emulsifier A is added to the solution). After stirring for 24 hours, the organic solvent was evaporated and removed. With the volatilization of the organic solvent, the inner core solidified into balls to form polymer nanoparticles, which were filtered through a 0.22 μm aqueous phase filter to obtain a transparent blank nanoparticle solution, which was stored at 4°C.
Embodiment 2
[0052] Embodiment 2: Preparation of paclitaxel-loaded polymer nanoparticles
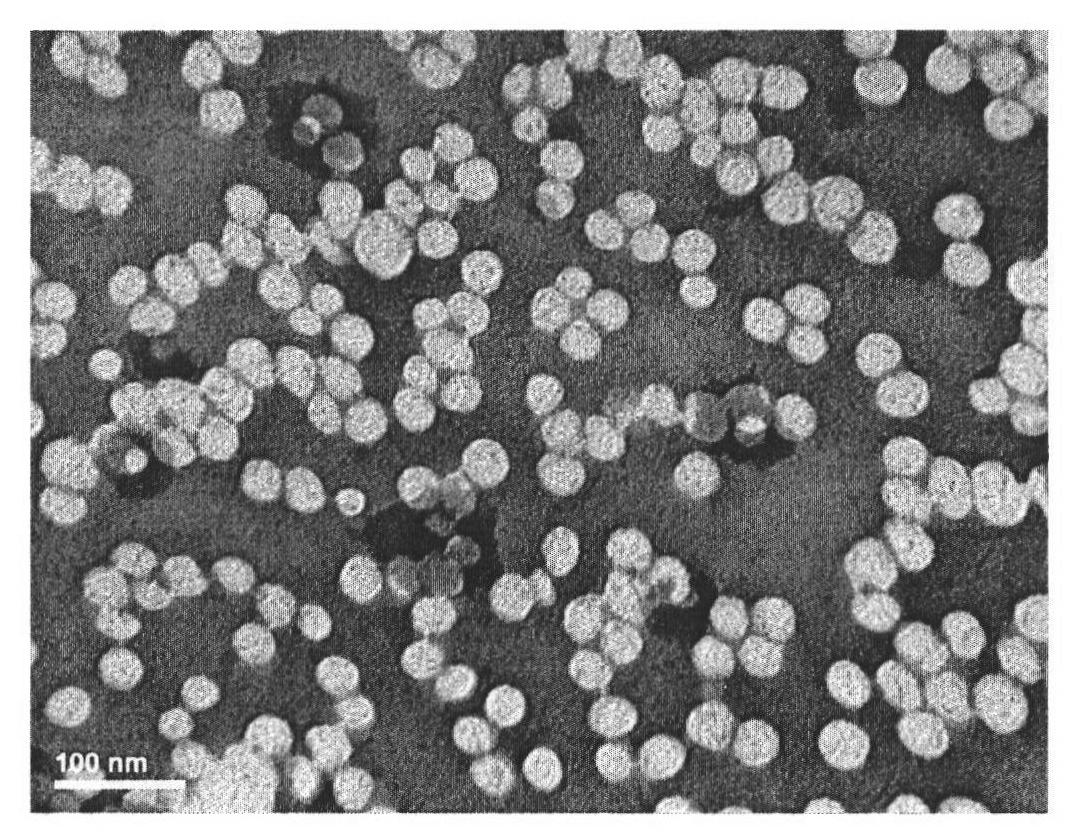
[0053] 8.0mg paclitaxel and 85.1mg polylactic acid-polyethylene glycol diblock copolymer, diblock molecular weight are 60,000 and 5,000 respectively, dissolve in 5mL acetone; Add acetone solution in the TPGS solution of 40mL 0.08%, then The mixture was processed under high pressure (30,000 psi) in a homogenizer (suitably sterilized) until a nanoemulsion was obtained; aseptically frozen to -30°C and lyophilized for 57 hours while warming to 35°C. The obtained powder containing 8.59% (w / w) paclitaxel was reconstituted with 0.9% sodium chloride aqueous solution to obtain a paclitaxel concentration of 2 mg / mL.
[0054] The obtained paclitaxel-encapsulated nano drug loading is 8.59 (w / w)%, the obtained preparation has an average value of 0.17 microns, pH 7.2-7.6, and stability greater than 24 hours.
Embodiment 3
[0055] Embodiment 3: Preparation of paclitaxel-loaded polymer nanoparticles
[0056] 10.1mg of paclitaxel and 67.8mg of poly(lactic acid-polyethylene glycol) diblock copolymer, the diblock molecular weights are 30,000 and 5,000 were dissolved in 4mL of acetone; acetone was added to 40mL of 0.05% TPGS solution, the mixture was under high pressure (30,000 psi) in a homogenizer (appropriately sterilized) until a nanoemulsion is obtained; freeze to -30°C under aseptic conditions and lyophilize for 57 hours while warming to 35°C. The obtained powder containing 12.96% (w / w) paclitaxel was reconstituted with 0.9% aqueous sodium chloride solution to obtain a paclitaxel concentration of 2 mg / mL.
[0057] The drug-loading capacity of the obtained paclitaxel-coated nanoparticles is 12.96%, and the obtained preparation has an average value of 0.16 microns, a pH of 7.2-7.6, and a stability of more than 24 hours.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com