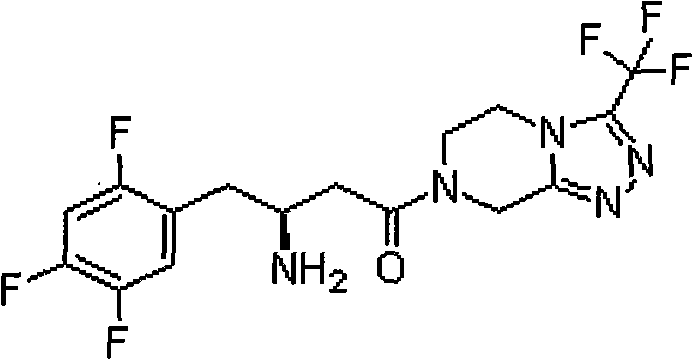
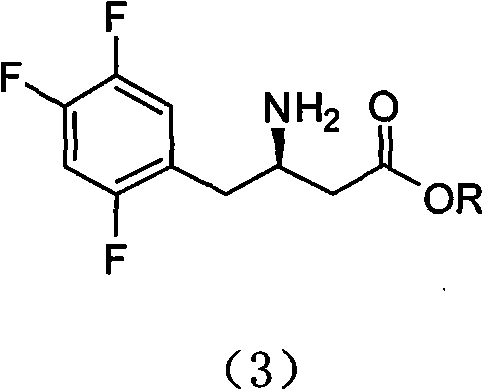
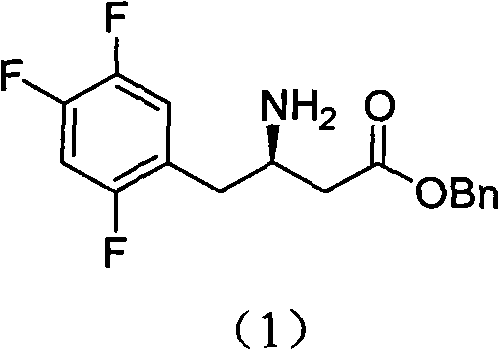
A kind of chiral resolution method of sitagliptin intermediate
A technology for splitting reagents and compounds, which is applied in the field of chiral splitting of sitagliptin intermediates, and can solve problems such as difficult salt formation, unsatisfactory splitting effect, and poor salt-forming effect of R-camphorsulfonic acid. , to achieve the effect of stable process, recyclable solvent and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Example 1: Preparation of compound (2)
[0026] 50 g (263 mmol) of 2,4,5-trifluorophenylacetic acid, 42 g of Meldrum's acid were added to 125 ml of acetonitrile, 2.6 g of DMAP and 94 ml of N,N-diisopropylethylamine were added with stirring, and the mixture was stirred to clear. 36ml of pivaloyl chloride was added dropwise at room temperature. After the addition, the temperature was raised to 50°C for reaction for 2 hours, cooled to 0°C, poured into 300ml of 1N hydrochloric acid solution, stirred for 30 minutes, and filtered. The filter cake was vacuum dried at 40°C to obtain 65 g of yellow solid. Add 65g of the yellow solid to 450ml of acetonitrile, add 23g of benzyl alcohol at room temperature, heat to reflux and react for 2 hours, then add 80g of ammonium acetate to continue the reaction for 2 hours, cool to 45°C, distill off the acetonitrile under reduced pressure, and then add ethyl acetate 500ml, water 300ml, saturated sodium carbonate 300ml, stirred for 15 minutes, ...
Embodiment 2
[0028] Example 2: Preparation of compound (1) resolving salt
[0029] 32.3g (100mmol) of benzyl 3-amino-4-(2,4,5-trifluorophenyl)butyrate was dissolved in 600ml of acetone, and 38.6g of D-di-p-toluoyltartaric acid was added in batches at room temperature ( 100mmol), stirred for 30 minutes, a large amount of solid precipitated, and then heated to reflux and stirred for 1 hour. Cool to room temperature and stir for 2 hours, filter, and directly beat the filter cake.
[0030] The filter cake obtained above was added to 400ml of acetone, heated to reflux and stirred for 1 hour. Cool to room temperature and stir for 2 hours, filter, add the obtained filter cake to 300ml of acetone, heat to reflux and stir for 1 hour. The temperature was lowered to room temperature and stirred for 2 hours, filtered, and dried under vacuum at 40°C to obtain 22 g (31 mmol) of D-di-p-toluoyl tartrate of compound (1) with a yield of 31%. Sampling and testing showed that the ee value reached more than 99%,...
Embodiment 3
[0031] Example 3: Preparation of compound (1) resolving salt
[0032] 32.3g (100mmol) of benzyl 3-amino-4-(2,4,5-trifluorophenyl)butyrate was dissolved in 600ml of acetone, and 35.8g (100mmol) of D-xyltoyltartaric acid was added in batches at room temperature. ), stirred for 30 minutes, a large amount of solids precipitated, and then heated to reflux and stirred for 1 hour. Cool to room temperature and stir for 2 hours, filter, and directly beat the filter cake.
[0033] The filter cake obtained above was added to 400ml of acetone, heated to reflux and stirred for 1 hour. Cool to room temperature and stir for 2 hours, filter, add the obtained filter cake to 300ml of acetone, heat to reflux and stir for 1 hour. The temperature was lowered to room temperature and stirred for 2 hours, filtered, and dried under vacuum at 40°C to obtain 17.7 g (26 mmol) of D-dyltoyl tartrate of compound (1) with a yield of 26%. Sampling and testing showed that the ee value reached more than 99% and t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com