Method for extracting high-purity 1,3-butadiene by using N-methylpyrrolidone
A technology of methylpyrrolidone and butadiene, applied in the direction of distillation purification/separation, etc., can solve the problems of not being able to obtain high-purity 1.3-butadiene, shortening contact time, and low single-pass conversion rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
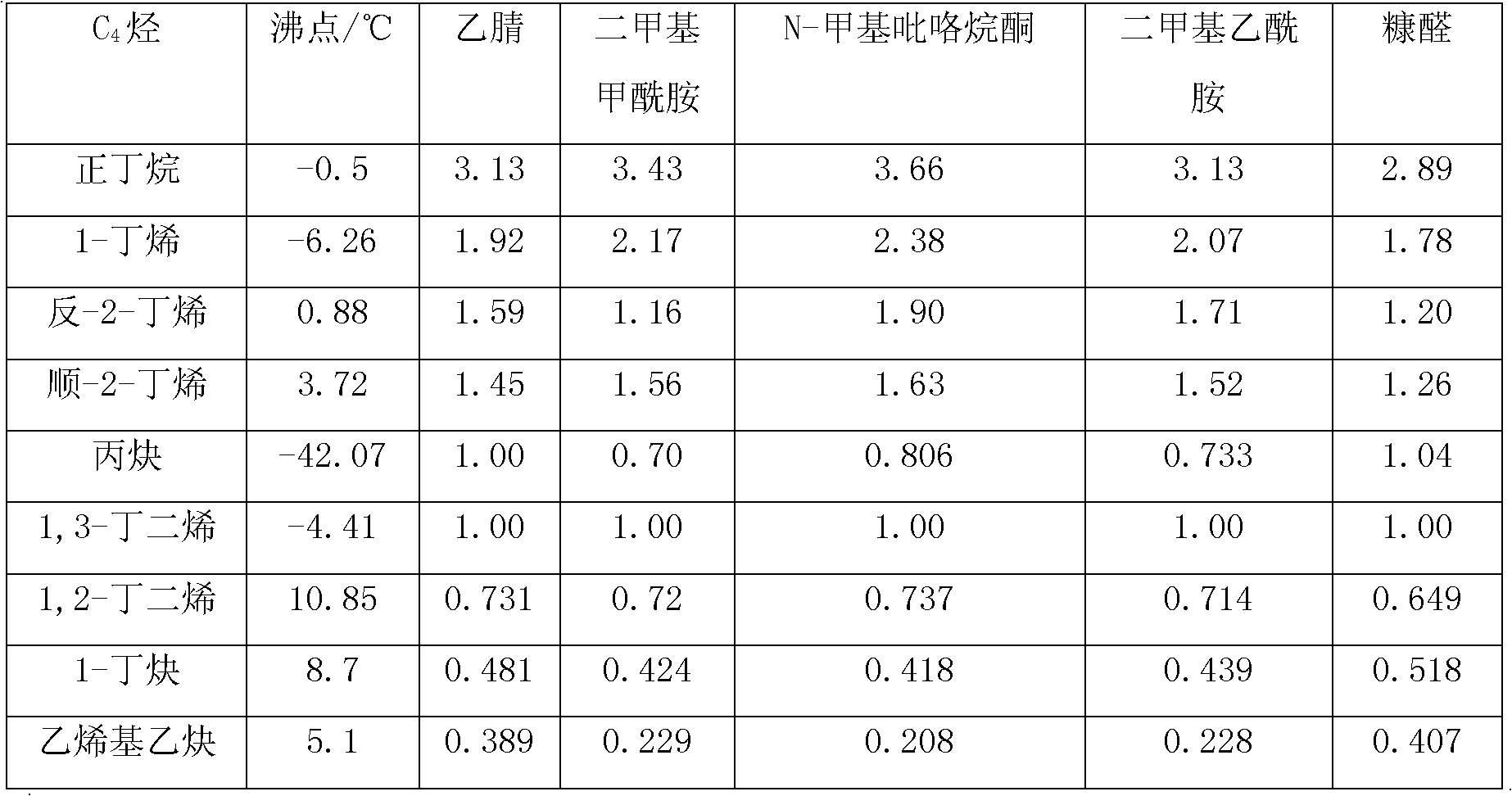
[0018] 1,3-Butadiene C 4 The distillate undergoes extractive distillation after selective hydrogenation to remove alkynes. The selective hydrogenation process is carried out in the liquid phase. The catalyst used is palladium loaded on alumina, and N-methylpyrrolidone is used as the solvent. It is absorbed twice and rectified twice to obtain a purity of 99.8%-99.9%, and the alkyne content is <50μg / g of polymer grade 1,3-butadiene.
Embodiment 2
[0020] The C4 fraction containing 1,3-butadiene is subjected to one-stage or two-stage extractive distillation by solvent extraction distillation; in the process of one-stage extractive distillation, the alkyne is first separated by ordinary distillation, and then the alkane is separated by extractive distillation And olefins, and then through precise distillation to obtain polymer grade 1,3-butadiene.
Embodiment 3
[0022] The C4 fraction containing 1,3-butadiene is subjected to selective hydrogenation to remove alkynes before extractive distillation; the selective hydrogenation process is carried out in the gas phase, and the catalyst used is loaded on alumina copper oxide; % of acetonitrile as a solvent, extraction and distillation separation of C4 alkanes and olefins, the extraction tower has 120 trays. After precise distillation, polymer grade 1,3-butadiene is obtained.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| boiling point | aaaaa | aaaaa |
| freezing point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com