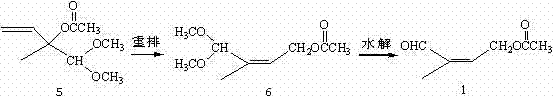
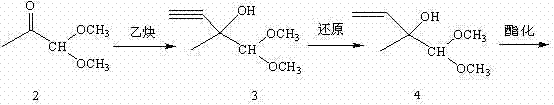
Method for preparing 4-acetoxy-2-methyl-2-butene-1-aldehyde
An acetoxy and methyl technology, applied in the field of preparation of key intermediates of vitamin A, can solve the problems of long route and complicated operation, and achieve the effects of high content and yield, mild reaction system and concise route
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Example 1: Preparation of 2-methyl-2-acetoxy-3-buten-1-al 7.
[0021] Add 1N hydrochloric acid aqueous solution (50ml) into a 250ml three-necked flask with a reflux condenser, keep it warm in an oil bath, and under magnetic stirring, add 5 (50g, 0.21mol) dropwise at 75-80°C, and the dropwise addition is completed in about 30 minutes, and continue to keep warm Stir for about 2 hours, the reaction is basically completed by gas chromatography, add toluene (100ml) for extraction, separate layers, wash the organic layer with aqueous sodium bicarbonate until neutral, dry over anhydrous magnesium sulfate, distill off the solvent under reduced pressure to obtain a crude product, the crude product is reduced to Pressure distillation, collecting 45~50℃ / 2.0mmHg fractions to obtain colorless transparent liquid 8 (28.9g, 91.3%), GC content 94.2%.
[0022] GC-MS(m / z): 143, 113, 71, 55, 43(100%); IR(ν / cm -1 ): 1744, 1235; 1 HNMR (CDCl 3 )δ: 1.544 (s, 3H); 2.171 (s, 3H); 5.338-5.438...
Embodiment 2
[0023] Example 2: Preparation of 4-acetoxy-2-methyl-2-butene-1-al 1 (pentacarbon aldehyde 1).
[0024] Add 7 (23.0g, 0.15mol), solvent acetonitrile (25ml) and bisacetonitrile palladium chloride (0.2g) into a 250ml three-necked flask, keep warm in an oil bath, and keep warm at 55-60°C for about 48h under magnetic stirring. Chromatography traced that the reaction was basically completed, the reaction solution was distilled under reduced pressure to remove the solvent, the crude product was distilled under reduced pressure, and the 70-75°C / 1.5mmHg fraction was collected to obtain a colorless transparent liquid 1 (18.6g, 80.7%) with a GC content of 92.3%. 1 HNMR (CDCl 3 )δ: 1.804 (s, 3H); 2.123 (s, 3H); 4.865-4.912 (m, 2H); 6.480-56.513 (m, 1H); 9.458 (s, 1H).
Embodiment 3
[0025] Example 3: Preparation of 2-methyl-2-acetoxy-3-buten-1-al 7.
[0026] Add 1N sulfuric acid aqueous solution (150ml) into a 250ml three-necked flask with a reflux condenser, keep it warm in an oil bath, and under magnetic stirring, add 5 (50g, 0.21mol) dropwise at 55-65°C, and the dropwise addition is completed in about 30 minutes, and continue to keep warm Stir for about 2 hours, the reaction is basically completed by gas chromatography, add toluene (100ml) for extraction, separate layers, wash the organic layer with aqueous sodium bicarbonate until neutral, dry over anhydrous magnesium sulfate, distill off the solvent under reduced pressure to obtain a crude product, the crude product is reduced to Pressure distillation, collecting 45~50℃ / 2.0mmHg fractions to obtain colorless transparent liquid 8 (24.9g, 78.0%), GC content 93.4%. The proton nuclear magnetic spectrum is identical with embodiment 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com