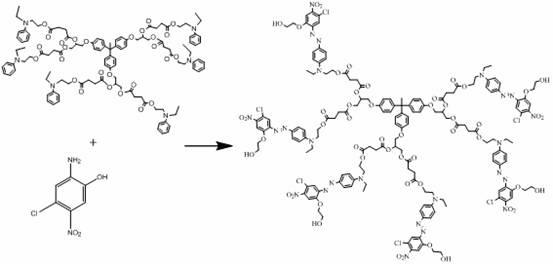
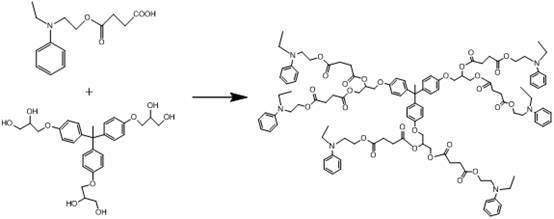
A kind of hexadendritic azosiloxane dye and its synthetic method
A technology of azo siloxane and synthesis method, applied in azo dyes, organic dyes, chemical instruments and methods, etc., can solve the problems of low thermal stability, difficult device application, etc., achieve good solubility, reduce static electricity, etc. Effects of interaction, enhanced loading concentration and polarization efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] R 1 for nitro, R 2 Be the synthetic method of the hexabranch azosiloxane dye of hydrogen, its step is as follows:
[0023] (1) Dissolve 1 mole of 1,1,1-tris(4-hydroxyphenyl)ethane in 600 mL of ethanol solution, add dropwise to an aqueous solution containing 2 moles of sodium hydroxide, reflux and stir for half an hour. Then 3 moles of 3-chloropropyl-1,2-diol were added, and the reflux reaction was continued for 4 hours. Cool after the reaction, pour the solvent into water, extract with ethyl acetate, and distill off the solvent under reduced pressure to obtain a dendritic nucleus containing propylene glycol. Its synthetic reaction formula is:
[0024]
[0025] NMR: 1 H NMR (500 MHz, DMSO- d 6 ): δ = 2.03 (s, 3H, C H 3 ), 3.43 (d, 6H, OC H 2 ), 3.80 (m, 6H, O H ), 3.95 (m, 3H, C H ), 4.67(d, 3H, C H 2 OH), 4.95 (d, 3H, C H 2 OH), 6.82 (d, 6H, Ar H ), 6.91 (d, 6H, Ar H ).
[0026] 13 C NMR (125 MHz, DMSO- d 6 ): δ = 157.167, 141.785, 129.695, 114....
Embodiment 2
[0044] R 1 for nitro, R 2 Be the synthetic method of the six branched azosiloxane dyes of chlorine, its step is as follows:
[0045] (1) Dissolve 1 mole of 1,1,1-tris(4-hydroxyphenyl)ethane in 600 mL of ethanol solution, add dropwise to an aqueous solution containing 5 moles of potassium hydroxide, reflux and stir for half an hour. Then 4 moles of 3-chloropropyl-1,2-diol were added, and the reflux reaction was continued for 4 hours. Cooling after the reaction is over, the solvent is poured into water, extracted with ethyl acetate, and the solvent is distilled off under reduced pressure to obtain a dendritic nucleus containing propylene glycol; its synthetic reaction formula is:
[0046]
[0047] NMR: 1 H NMR (500 MHz, DMSO- d 6 ): δ = 2.03 (s, 3H, C H 3 ), 3.43 (d, 6H, OC H 2 ), 3.80 (m, 6H, O H ), 3.95 (m, 3H, C H ), 4.67(d, 3H, C H 2 OH), 4.95 (d, 3H, C H 2 OH), 6.82 (d, 6H, Ar H ), 6.91 (d, 6H, Ar H ).
[0048] 13 C NMR (125 MHz, DMSO- d 6 ): δ = 157...
Embodiment 3
[0064] R 1 for hydrogen, R 2 Be the synthetic method of the six branched azosiloxane dyes of nitro, its step is as follows:
[0065] (1) Dissolve 1 mole of 1,1,1-tris(4-hydroxyphenyl)ethane in 600 mL of ethanol solution, add dropwise to an aqueous solution containing 5 moles of sodium hydroxide, reflux and stir for half an hour. Then 5 moles of 3-chloropropyl-1,2-diol were added, and the reflux reaction was continued for 4 hours. Cool after the reaction, pour the solvent into water, extract with ethyl acetate, and distill off the solvent under reduced pressure to obtain a dendritic nucleus containing propylene glycol. Its synthetic reaction formula is:
[0066] NMR: 1 H NMR (500 MHz, DMSO- d 6 ): δ = 2.03 (s, 3H, C H 3 ), 3.43 (d, 6H, OC H 2 ), 3.80 (m, 6H, O H ), 3.95 (m, 3H, C H ), 4.67(d, 3H, C H 2 OH), 4.95 (d, 3H, C H 2 OH), 6.82 (d, 6H, Ar H ), 6.91 (d, 6H, Ar H ).
[0067] 13 C NMR (125 MHz, DMSO- d 6 ): δ = 157.167, 141.785, 129.695, 114.142,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com