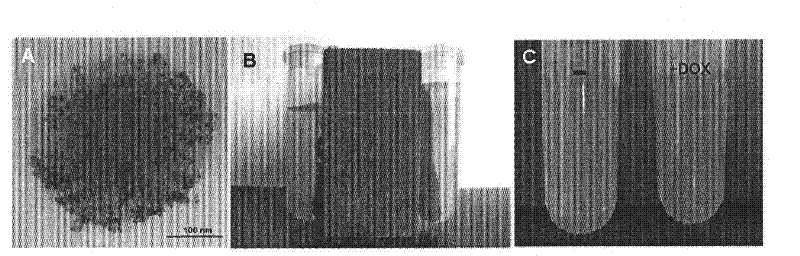
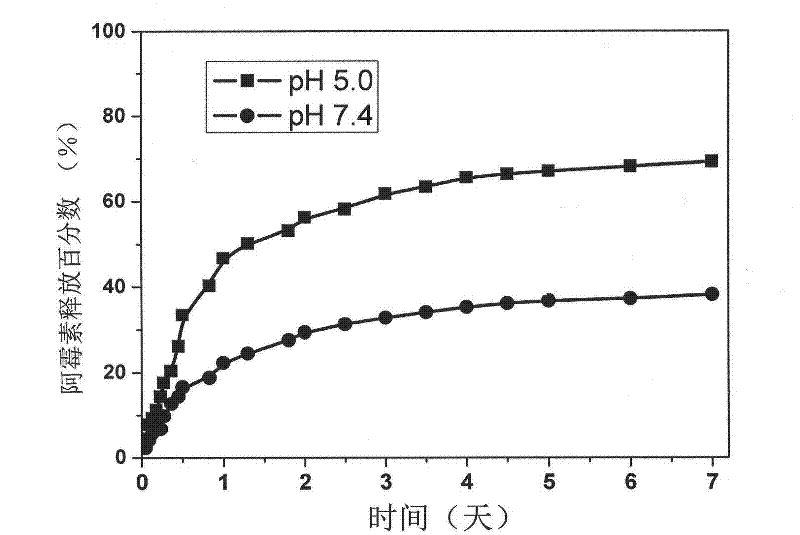
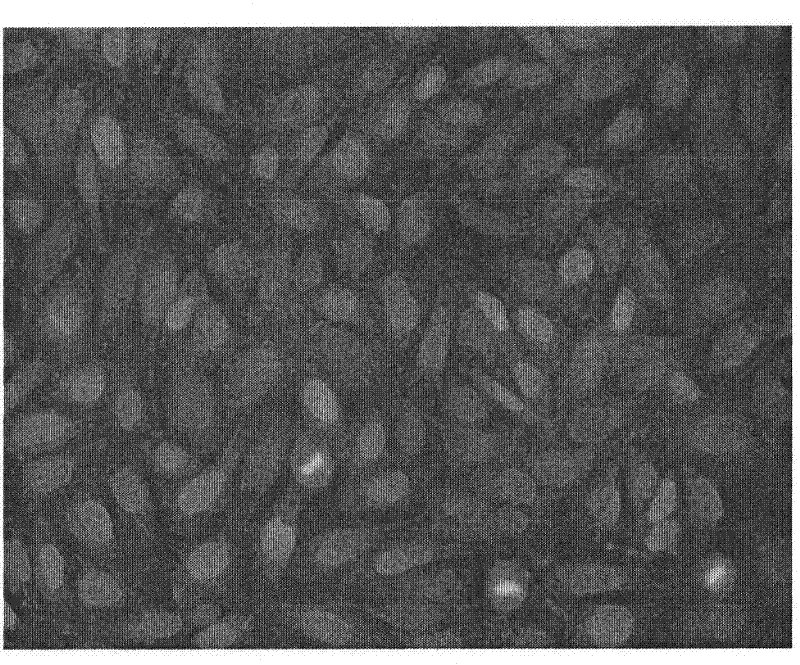
Preparation method and application of a drug-loaded magnetic composite nanomaterial
A magnetic composite nanometer and magnetic nanoparticle technology, which is applied in the direction of medical formulas, unknown raw materials, medical preparations with non-active ingredients, etc., can solve problems such as the inability of drugs to concentrate tumor cells, failure of tumor treatment, and large toxic and side effects. Overcome multi-drug resistance, improve toxic and side effects, and improve curative effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] 1. The phacoemulsification method is used to prepare a multifunctional magnetic composite nanomaterial integrating magnetism, sustained release and treatment.
[0042] ① Synthesis of Fe by co-precipitation method 3 o 4 Magnetic nanoparticles, and then coated with oleic acid, the particle size is 8nm;
[0043] ②Extract 0.1mg / ml doxorubicin with triethylamine, and the molar ratio of doxorubicin to triethylamine is 1:3;
[0044] 3. prepare the dichloromethane solution of polylactic acid / glycolic acid copolymer (PLGA), the PLGA concentration is 5mg / ml;
[0045] ④ The Fe obtained in step ① 3 o 4 Add the magnetic material to the PLGA dichloromethane solution prepared in step 3, then add the mixed solution of adriamycin extracted by step 2 triethylamine, mix well, and make the Fe 3 o 4 The final concentration of magnetic nanoparticles is 5 mg / ml, and the final concentration of doxorubicin is 0.05 mg / ml;
[0046] ⑤ Add 2ml of the mixed solution obtained in step ④ dropwis...
Embodiment 2
[0052] 1. The phacoemulsification method is used to prepare a multifunctional magnetic composite nanomaterial integrating magnetism, sustained release and treatment.
[0053] ① Synthesis of Fe by co-precipitation method 3 o 4 Magnetic nanoparticles, and then coated with oleic acid, the particle size is 8nm;
[0054] ②Extract 0.5 mg / ml doxorubicin (DOX) with triethylamine, and the molar ratio of doxorubicin to triethylamine is 1:3;
[0055] 3. prepare the dichloromethane solution of polylactic acid / glycolic acid copolymer (PLGA), the PLGA concentration is 30mg / ml;
[0056] ④ The Fe obtained in step ① 3 o 4 Add the magnetic material to the PLGA dichloromethane solution prepared in step 3, then add the mixed solution of adriamycin extracted by step 2 triethylamine, mix well, and make the Fe 3 o 4 The final concentration of magnetic nanoparticles is 10 mg / ml, and the final concentration of doxorubicin is 0.25 mg / ml;
[0057] ⑤ Add 2ml of the mixed solution obtained in step ...
Embodiment 3
[0064] 1. The phacoemulsification method is used to prepare a multifunctional magnetic composite nanomaterial integrating magnetism, sustained release and treatment.
[0065] ① Synthesis of Fe by co-precipitation method 3 o 4 Magnetic nanoparticles, and then coated with oleic acid, the particle size is 8nm;
[0066] ② Extract 1mg / ml doxorubicin with triethylamine, the molar ratio of doxorubicin to triethylamine is 1:3;
[0067] 3. prepare the dichloromethane solution of polylactic acid / glycolic acid copolymer (PLGA), the PLGA concentration is 100mg / ml;
[0068] ④ The Fe obtained in step ① 3 o 4 Add the magnetic material to the PLGA dichloromethane solution prepared in step 3, then add the mixed solution of adriamycin extracted by step 2 triethylamine, mix well, and make the Fe 3 o 4 The final concentration of magnetic nanoparticles is 5 mg / ml, and the final concentration of doxorubicin is 0.5 mg / ml;
[0069] ⑤ Add 2ml of the mixed solution obtained in step ④ dropwise to...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com