Paclitaxel alhumin submicron for injection and preparation method thereof
A technology of paclitaxel and albumin, which is applied in the directions of pharmaceutical formulations, non-active ingredients of polymer compounds, powder delivery, etc., can solve the problems of organic solvent residue, complicated preparation process, high preparation cost, etc., and achieve reduction of preparation cost, simple preparation process, The effect of convenient clinical use
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
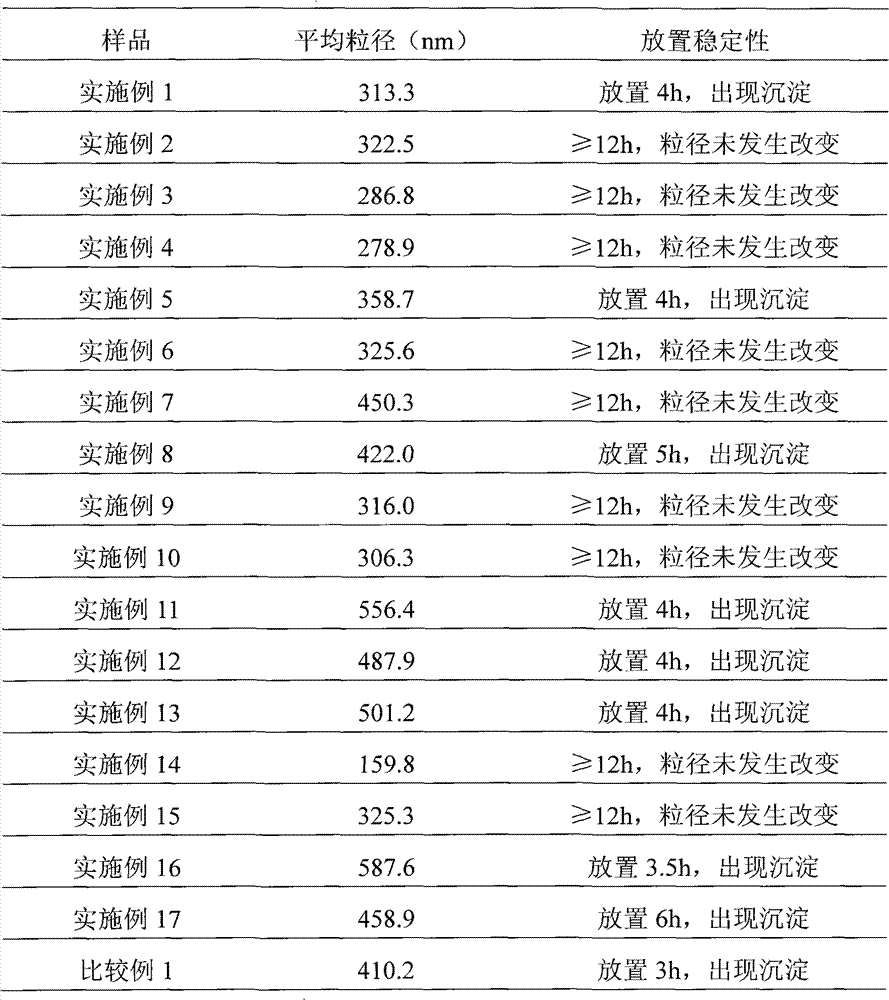
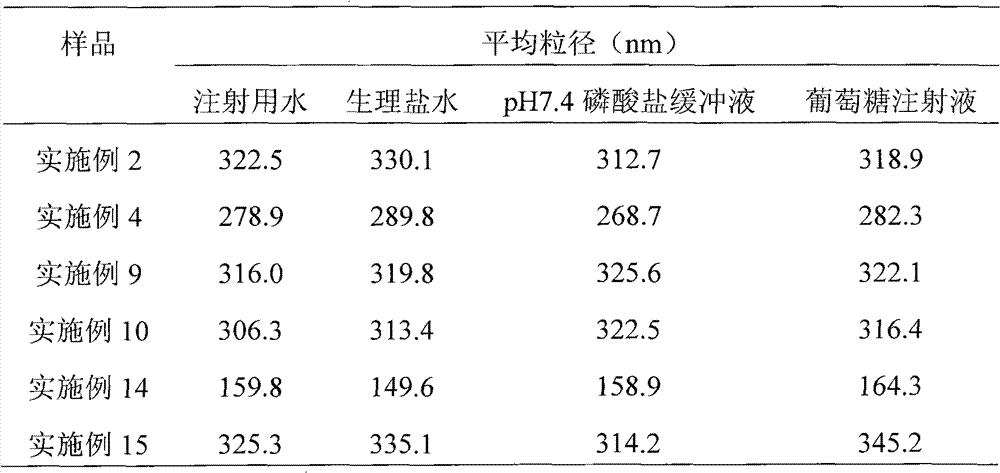
[0039] Weigh 50 mg of paclitaxel, dissolve in 16 ml of tert-butanol as the organic phase; weigh 400 mg of human serum albumin, 200 mg of mannitol, and 200 mg of glycine, dissolve in 24 ml of water for injection as the water phase. The organic phase and the aqueous phase were mixed evenly under the condition of stirring (500 rpm), dissolved and clarified, filtered and sterilized through a 0.22 μm microporous membrane, and distributed into clean vials. The vial is frozen at a temperature of -30°C to -50°C, lyophilized in a freeze dryer to remove tert-butanol and moisture, filled with nitrogen, stoppered, and capped to obtain the product.
Embodiment 2
[0041]Weigh 20 mg of paclitaxel, dissolve in 20 ml of tert-butanol as the organic phase; weigh 400 mg of human serum albumin, 100 mg of glucose, dissolve in 80 ml of water for injection as the water phase. The organic phase and the aqueous phase were mixed evenly under stirring (manual stirring), dissolved and clarified, filtered and sterilized through a 0.22 μm microporous membrane, and then packed into clean vials. The vial is frozen at a temperature of -30°C to -50°C, lyophilized in a freeze dryer to remove tert-butanol and moisture, filled with nitrogen, stoppered, and capped to obtain the product.
Embodiment 3
[0043] Weigh 20 mg of paclitaxel, dissolve in 20 ml of tert-butanol as the organic phase; weigh 600 mg of human serum albumin, 500 mg of glycine, dissolve in 60 ml of water for injection as the water phase. The organic phase and the aqueous phase were mixed evenly under the condition of stirring (1500 rpm), dissolved and clarified, filtered and sterilized through a 0.22 μm microporous membrane, and distributed into clean vials. The vial is frozen at a temperature of -30°C to -50°C, lyophilized in a freeze dryer to remove tert-butanol and moisture, filled with nitrogen, stoppered, and capped to obtain the product.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com