Novel method for preparing tulathromycin
A technology of telamycin and norazithromycin, which is applied to the preparation of sugar derivatives, chemical instruments and methods, and the production of bulk chemicals, and can solve problems such as complicated operation process and unfavorable industrial production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
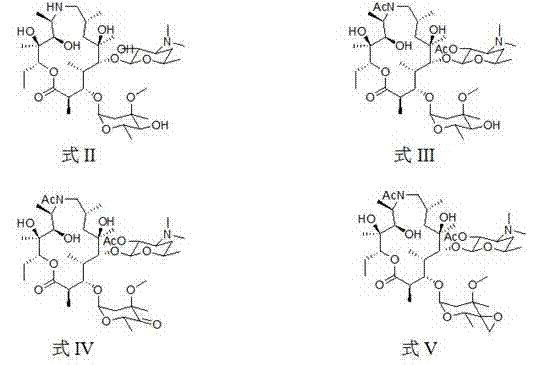
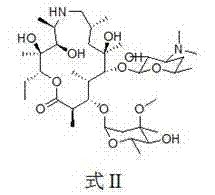
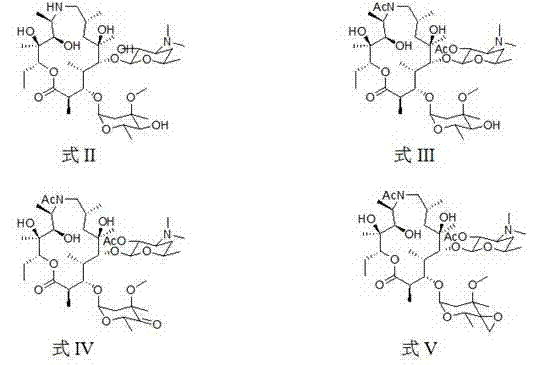
[0057] (2R,3S,4R,5R,8R,10R,11R,12S,13S,14R)-13-[[2,6-dideoxy-3-C-methyl-3-O-methyl-α-L -nucleo-hexapyranosyl]-oxy]2-ethyl-3,4,10-trihydroxy-3,5,8,10,12,14-hexamethyl-11-[[3,4, 6-trideoxy-3-(dimethylamino)-2-O-acetyl-β-D-xyl-hexapyranosyl]oxy]-1-oxa-6-azacyclopentadecane (15 -Yuan macrocycle) (intermediate one) preparation
[0058] Add 7.34 g (0.01 mol) of norazithromycin to a 250 ml round bottom flask, dissolve it with 40 ml of dichloromethane and place it in an ice bath, then add 1.4 ml of triethylamine (0.01 mol), and then add 1.5 ml of triethylamine after 20 minutes ml acetic anhydride (0.015 mol), react in the ice bath for 20 minutes, then remove the ice bath, react at room temperature for 10 hours, then add 1.0 ml acetic anhydride (0.01 mol), react for another 8 hours, stop the reaction, pour into the reaction system Add 50 ml of 1M sodium dihydrogen phosphate solution, stir at room temperature for 30 minutes, separate the organic phase, then extract the aqueous phase 3...
Embodiment 2
[0062] (2R,3S,4R,5R,8R,10R,11R,12S,13S,14R)-13-[[2,6-dideoxy-3-C-methyl-3-O-methyl-4-oxo Substituent-α-L-nucleo-hexapyranosyl]-oxy]2-ethyl-3,4,10-trihydroxyacid-3,5,8,10,12,14-hexamethyl-11- [[3,4,6-trideoxy-3-(dimethylamino)-2-O-acetyl-β-D-xyl-hexapyranosyl]oxy]-1-oxo-6-aza Preparation of cyclopentadecane (15-membered macrocycle) (intermediate 2)
[0063] Add 2.05 g (25 mmol) of intermediate formula II to a 100 ml single-necked round bottom flask, dissolve it with 25 ml of dry dichloromethane at room temperature, add 532 ul dimethyl sulfoxide (75 mmol), stir well and place React at low temperature for 15 minutes, then add 1.5 ml trifluoroacetic anhydride (0.0106 mol) dropwise, continue to react for 1 hour, add 3 ml triethylamine (0.021 mol), stir at low temperature for 30 minutes, then naturally warm to room temperature, close to room temperature Add 30 ml of saturated NaCl solution, after 30 minutes, separate the organic phase, extract the aqueous phase with chloroform 5 t...
Embodiment 3
[0067] (2R,3S,4R,5R,8R,10R,11R,12S,13S,14R)-13-[[2,6-dideoxy-3-C-methyl-3-O-methyl-4-C -[epoxymethyl]-α-L-nucleo-hexapyranosyl]-oxy]2-ethyl-3,4,10-trihydroxy-3,5,8,10,12,14-hexa Methyl-11-[[3,4,6-trideoxy-3-(dimethylamino)-2-O-acetyl-β-D-xyl-hexapyranosyl]oxy]-1-oxa - Preparation of 6-azacyclopentadecane (15-membered macrocycle) (intermediate 3)
[0068] Add 1.38 g (0.008 mol) of trimethylsulfur bromide, 25 ml of dry tetrahydrofuran and 8.8 ml of 1M potassium hexamethyldisilazide (0.008 mol) into a 100 ml single-necked round bottom flask, place at -15 After reacting for 1 hour at low temperature under the protection of argon, add 10ml tetrahydrofuran dissolved in 2.04 g (0.0025 mol) of intermediate 3 to the reaction system, react at low temperature for 30 minutes, transfer to room temperature, and use 20 ml saturated chlorinated The ammonium solution was extracted, stirred at room temperature for 30 minutes, and the organic phase was separated, the aqueous phase was extracte...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com