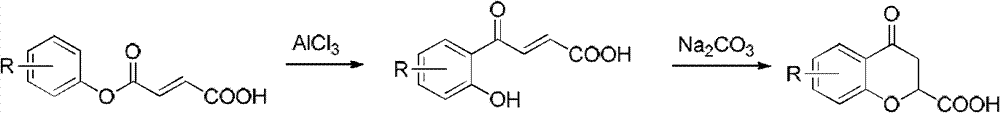
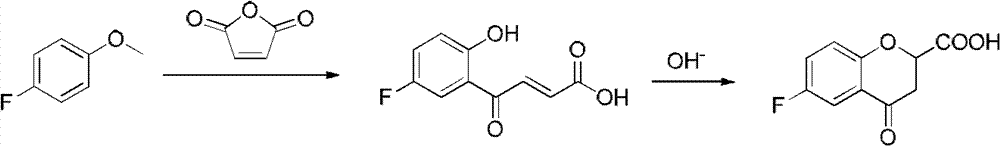
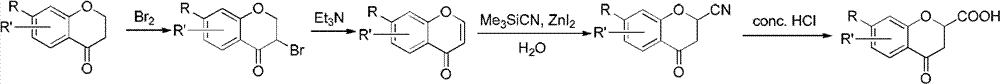Method for synthesizing 6-fluoro-3,4-dihydro-2H-1-benzopyran-2-carboxylic acid and alkyl ester thereof
A technology for the synthesis of alkyl and synthetic routes, which is applied in the field of synthesis of 6-fluoro-3,4-dihydro-2H-1-chromene-2-carboxylic acid and its alkyl esters, and can solve the problem of long steps problems, to achieve the effect of mild conditions, high yield and easy access to raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047]
[0048] Red phosphorus (4.8 g, 0.16 mol) was dissolved in gamma-butyrolactone (600.0 g, 6.96 mol). Heat and stir to raise the temperature to 100° C., and start to add liquid bromine (1124.0 g, 7.00 mol) dropwise. After the completion of the reaction was tracked by GC, the system was cooled to 10°C in an ice bath, and 1200 mL of methanol and 4 mL of concentrated sulfuric acid were added. Stir overnight at room temperature. The pH value of the system was adjusted to neutral with saturated sodium bicarbonate solution, and dichloromethane was added for extraction. The organic phase was dried by adding anhydrous sodium sulfate. Distillation after filtration gave the product 2,4-dibromobutyric acid methyl ester as 1400.0 g of light yellow liquid with a purity of 99.4% and a yield of 76.9%.
Embodiment 2
[0050]
[0051]Red phosphorus (4.8 g, 0.16 mol) was dissolved in gamma-butyrolactone (600.0 g, 6.96 mol). Heat and stir to raise the temperature to 100° C., and start to add liquid bromine (1124.0 g, 7.00 mol) dropwise. After the completion of the reaction was tracked by GC, the system was cooled to 10°C in an ice bath, and 1200 mL of ethanol and 4 mL of concentrated sulfuric acid were added. Stir overnight at room temperature. The pH value of the system was adjusted to neutral with saturated sodium bicarbonate solution, and dichloromethane was added for extraction. The organic phase was dried by adding anhydrous sodium sulfate. Distillation after filtration gave the product ethyl 2,4-dibromobutyrate as 1470.0 g of light yellow liquid with a purity of 99.1% and a yield of 76.4%.
Embodiment 3
[0053]
[0054] Sodium hydride (90.0 g, 2.25 mol) was dissolved in 1500 mL of benzene, and p-fluorophenol (224.0 g, 2.00 mol) was slowly added. Stir overnight at 40°C. The methyl 2,4-dibromobutyrate obtained in Example 1 was dissolved in 1500 mL of benzene, and the temperature was raised to 60°C. Slowly drop in the previously prepared sodium p-fluorophenolate solution and stir for 5 hours. Suction filtration, the filtrate was washed with water, and dried by adding anhydrous sodium sulfate. After filtration, distillation gave the product 4-bromo-2-(4-fluorophenoxy)butanoic acid methyl ester, which was 476.0 g of a colorless liquid, with a purity of 93.6% and a yield of 81.0%. 1 H-NMR (DCCl 3 , 500Hz): 7.01-6.96(m, 4H), 4.85(t, J=10, 1H), 4.51-4.46(m, 1H), 4.35-4.296(m, 1H), 3.75(s, 3H), 2.71 -2.64(m, 1H), 2.48-2.40(m, 1H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com