Preparation method and application of superfine calcium hexaboride
A technology of fine calcium hexaboride and calcium borate, which is applied in the field of preparation of ultrafine calcium hexaboride, can solve problems such as no reports of calcium boride, and achieve the effect of not being easy to sinter
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
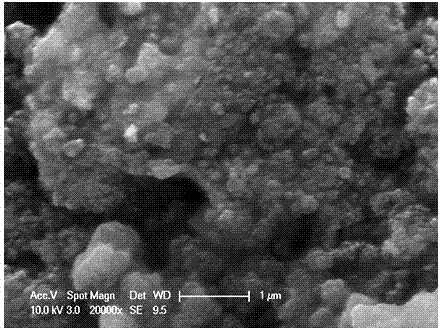
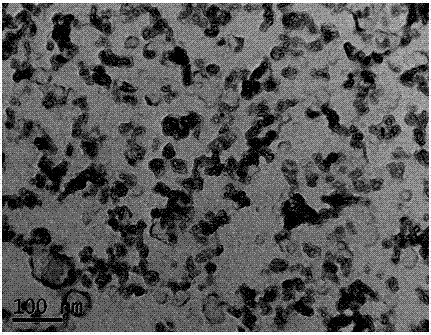
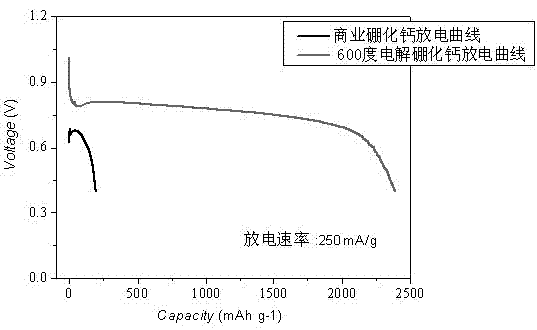
[0026] Heat CaO, boric acid and water in a water bath at 100°C for 2 hours, then filter to obtain white precipitated calcium borate powder, then press the obtained calcium borate powder into a solid porous test piece, sinter at 400-500°C for 2 hours to shape, The porous calcium borate test piece is placed in a stainless steel basket as the cathode, graphite as the anode, and CaCl at 500-600°C 2 -Electrolyze in LiCl electrolyte for 20 hours under 2.6-3.6V tank pressure, wash off the salt and impurities in the product with water and dilute hydrochloric acid, and dry to obtain an ultrafine calcium hexaboride powder with a yellow appearance and a particle size of about 50nm ( figure 1 , 2 shows the obtained SEM and TEM pictures). After continuous electrolysis for 60 hours, put borax into the molten salt electrolyte and stir the electrolyte, and restart electrolysis after standing still. After repeating this many times, stop the electrolysis, dissolve the upper layer of calcium ch...
Embodiment 2
[0028] Ca(OH) 2 , borax and water were ball milled at room temperature for 2 hours, the product after ball milling was washed with water, filtered and dried to obtain a white powder, and the white powder was pressed into a porous test piece on a tablet machine, and then put into a muffle furnace at 650°C Calcined for 3 hours, the burnt calcium borate test piece was connected to the molybdenum wire as the cathode, tin dioxide as the anode at 600-690°C CaCl 2 -NaCl electrolyte with 2.8-3.8V cell piezo electrolysis for 12 hours, washing off the salt and impurities in the cathode product with water and dilute hydrochloric acid to obtain ultrafine calcium hexaboride with a particle size between 100nm and 1μm.
Embodiment 3
[0030] CaCl 2 , sodium borate and water were heated and reacted in a water bath at 100°C for 2 hours, and then filtered to obtain white precipitated calcium borate powder. Calcium borate and molybdenum mesh composite as solid cathode, graphite as anode, CaCl at 700-750°C under 2.4V-3.6V cell pressure 2 -Electrolyze in NaCl molten salt for 10 hours, remove the salt and a small amount of impurities from the product obtained at the cathode with water and dilute hydrochloric acid, and obtain CaB with a particle size of 1-5 μm 6 powder.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com