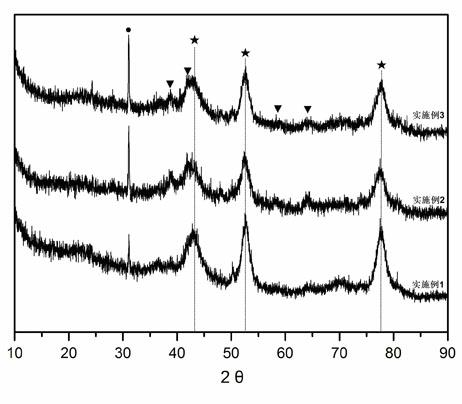
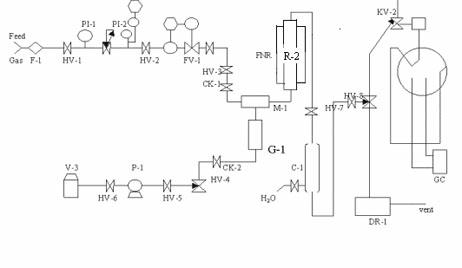
CO (carbon monoxide) sulfur tolerant shift catalyst applicable to high pressure process and preparation method thereof
A sulfur-resistant conversion and catalyst technology, which is applied in chemical instruments and methods, catalytic treatment of combustible gases, physical/chemical process catalysts, etc., can solve the problems of high low-temperature activity, easy loss of potassium, etc., and achieve good thermal stability. The effect of waste water discharge and low activation temperature
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0029] A method for preparing a CO sulfur-tolerant shift catalyst suitable for a high-pressure process comprises the following steps:
[0030] (1) Mix modified bauxite powder, pseudo-boehmite or aluminum nitrate with CeO 2 After the precursors of the mixture are mixed, they are kneaded with magnesium oxide or magnesium hydroxide to form a mixture;
[0031] (2) Add peptizer, binder, pore-forming agent, extrusion aid to the mixture obtained in step (1), and knead;
[0032] (3) drying the mixture obtained in step (2), extruding, and roasting to obtain the carrier of the catalyst;
[0033] (4) The catalyst carrier obtained in step (3) is impregnated with the proportioned catalyst active components CoO and MoO 3 , after drying and roasting, the finished catalyst is obtained.
[0034] The peptizer in the step (2) is a mixture of one or two of nitric acid and malonic acid, and its addition amount is 5-30% of the total mass of the carrier.
[0035] The binder in the step (2) is a mi...
Embodiment 1
[0041] Preparation of the carrier: 1097g of pseudoboehmite powder and 110g of modified bauxite powder were mixed evenly, and 6.5 grams of cerium nitrate (CeO 2 Accounting for about 0.3% of the total mass) dissolved in 400mL of 20% nitric acid, then added to the mixed powder together to form a gel, then added 360g of magnesium hydroxide and kneaded evenly, added 42g of squid powder, 24g of titanate, 40 g of citric acid was kneaded for 1 hour. The resulting slurry was dried at 110° C. for 1 hour, kneaded uniformly in a kneader, and extruded. The strip was calcined at 550° C. for 4 hours to obtain a catalyst carrier.
[0042] Catalyst preparation: impregnation with equal volume impregnation method, cobalt nitrate containing 3% CoO and MoO containing 8% 3 Ammonium molybdate was prepared as a complex solution, added to the prepared carrier, dried at 120°C for 2 hours, and calcined at 400°C for 2 hours to obtain the finished catalyst.
Embodiment 2
[0044] Preparation of the carrier: 877g of pseudoboehmite powder and 439g of modified bauxite powder were mixed evenly, and 15 grams of cerium nitrate (CeO 2 Accounting for about 1.0% of the total mass) dissolved in 300mL of 30% nitric acid, then added to the mixed powder together to form a gel, then added 295g of magnesium oxide and kneaded evenly, then added 40g of squash powder, 20g of boric acid, 40g of citric acid Knead for 1 hour. The resulting slurry was dried at 80° C. for 1 hour, then kneaded uniformly in a kneader, and extruded. The strip was calcined at 600° C. for 2 hours to obtain a catalyst carrier.
[0045] Catalyst preparation: Cobalt oxalate containing 3% CoO and MoO containing 8% 3 Ammonium molybdate was prepared as a complex solution, added to the prepared carrier, dried at 120°C for 2 hours, and calcined at 400°C for 2 hours to obtain the finished catalyst.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Specific surface area | aaaaa | aaaaa |
| Specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com