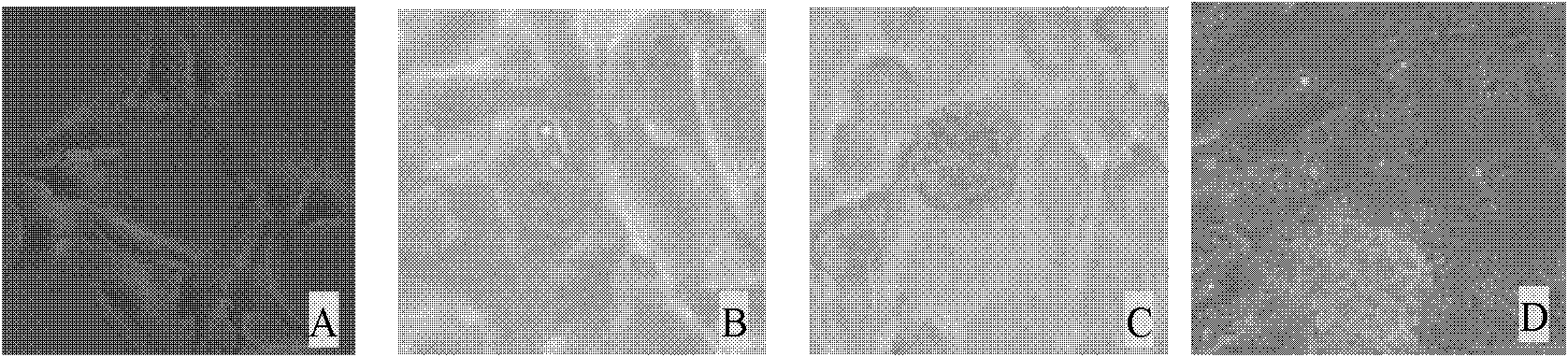Coccidia cell culture model and application thereof
A cell culture and cell technology, applied in the direction of embryonic cells, animal cells, vertebrate cells, etc., to achieve the effect of strong invasion ability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Example 1 Collection and purification of Eimeria tenella sporozoites
[0036] The purification of oocysts refers to the separation and purification technology of coccidia oocysts in the "Advanced Parasitology Experiment Guide", with slight modifications during the experimental operation (Suo Xun et al., 2006).
[0037] (1) The sporulated oocysts of Eimeria tenella were pressed by 1×10 4 Inoculate 14-day-old coccidial-free chicks per piece / only. On the 8th day after infection, the chickens were dissected and the cecum was collected to collect unsporulated oocysts. They were cultured at 28°C until they were fully sporulated, and the sporulated oocysts were collected and stored at 4°C. The period is 1-2 months.
[0038] (2) The sporulated oocysts of E.tenella stored at 4°C were centrifuged at 2500r / min for 10min at room temperature. After removing potassium dichromate, add an appropriate amount of distilled water to the precipitate, suspend the precipitate, centrifuge at...
Embodiment 2
[0045] The establishment of embodiment 2 Eimeria tenella DF-1 cell culture model
[0046] Digest well-growing DF-1 cells with 0.25% trypsin, collect the cells after 2-4 minutes, count with a cell counting plate, dilute with 10% DMEM, and dilute at 1×10 5 Spread 24-well plates at 37°C, 5% CO 2 Cultured for 24h (see figure 1 A), each hole access 1 × 10 5 Sporozoites, 41°C, 5% CO 2 Culture, it can be seen that sporozoites can invade DF-1 cells within 24h (such as figure 1 B), developed into the 1st generation schizont within 60h (see figure 1 C), at 72h, a large number of free first-generation merozoites can be observed in the cell supernatant (see figure 1 D), a small number of merozoites can continue to invade DF-1 cells, but no merozoites continue to develop into oocysts.
Embodiment 3
[0047] Example 3 Application of the Eimeria tenella DF-1 cell model in establishing a detection method for sporozoite invasion activity
[0048] 1. Establishment of detection method for invasion activity of Eimeria tenella sporozoites
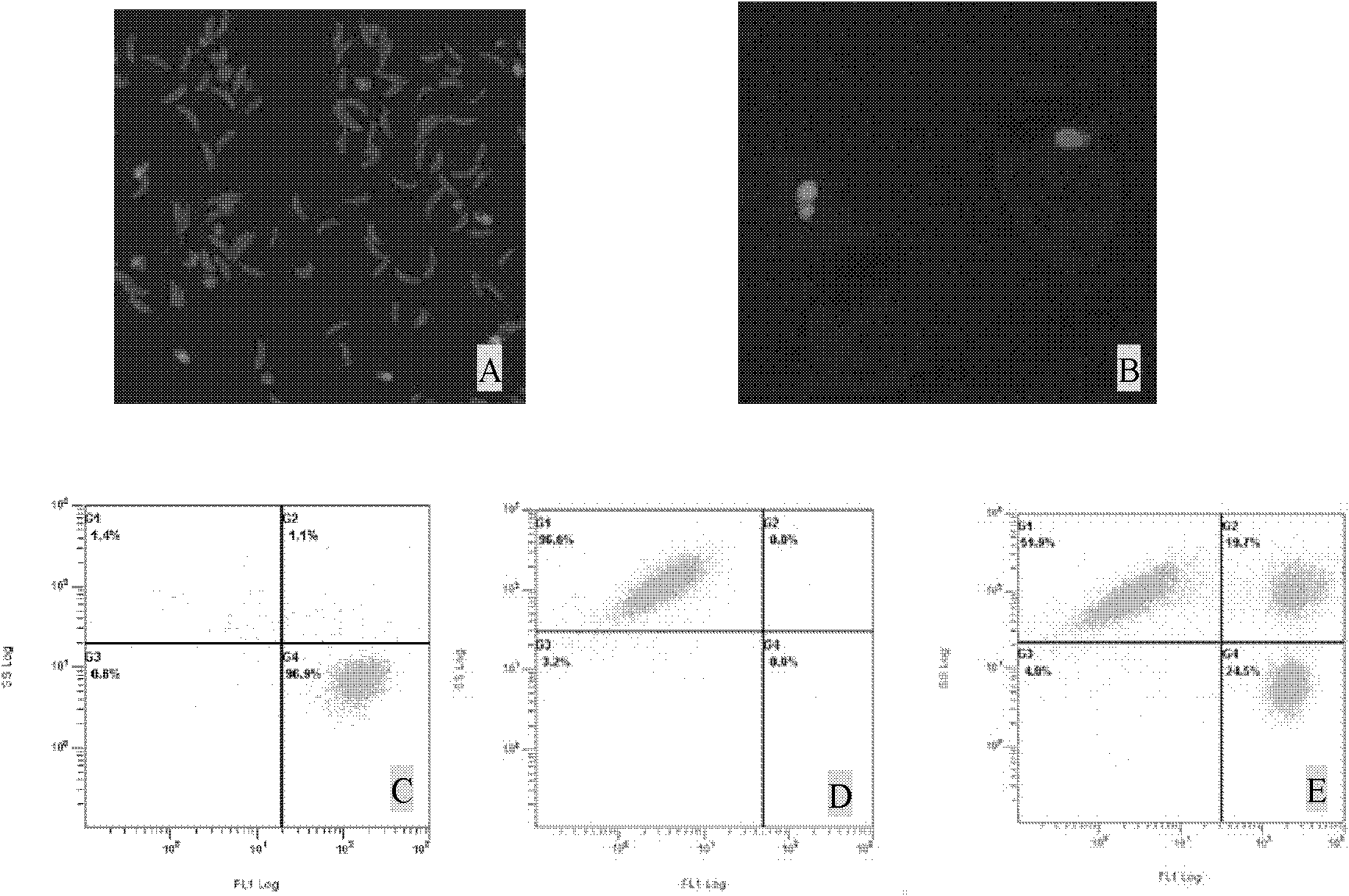
[0049] Count the purified sporozoites of Eimeria tenella, label the sporozoites according to the CFDA SE cell proliferation and tracer detection kit operation steps, inoculate DF-1 cell culture, wash twice with PBS before detection, trypsinize and collect Cells, detect the invasion cell number of coccidia sporozoites with flow cytometer, thereby calculate the invasion rate of sporozoites, the formula is: the invasion rate of sporozoites=100%×[invasion cell number / (non-invasion cell number+invasion cell number)]. Normal DF-1 cells and sporozoites labeled with CFDA SE (fluorescent probe 5(6)-carboxyfluorescein diacetate succinimidyl ester) were used as control groups. It can be seen that the fluorescence of sporozoites labeled with CFDA SE is v...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com