Human monoclonal antibody against EGFR, preparation method and purpose thereof
A fully human and antibody technology, applied in the biological field, can solve problems such as low affinity and failure to achieve humanization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0020] Preparation of Example Antibodies
[0021] (1) Cloning of human antibody light and heavy chain constant region genes
[0022] Lymphocyte separation fluid (product of Dingguo Biotechnology Development Co., Ltd.) was used to separate healthy human lymphocytes, and total RNA was extracted with Trizol reagent (product of Invitrogen Company). , 1982, 10: 4071-4079) reported sequences, respectively designed primers and used RT-PCR reaction to amplify the antibody heavy chain and light chain constant region genes. The PCR product was purified and recovered by agarose gel electrophoresis and cloned into the pGEM-T vector (promega company product). After sequencing verification, it was confirmed that the correct clone was obtained. SEQ ID NO: 1 and SEQ ID NO: 2 show the heavy chain constant region (C H ) nucleotide sequence and amino acid sequence. SEQ ID NO: 3 and SEQ ID NO: 4 show the light chain constant region (C L ) nucleotide sequence and amino acid sequence. The corr...
experiment example 1
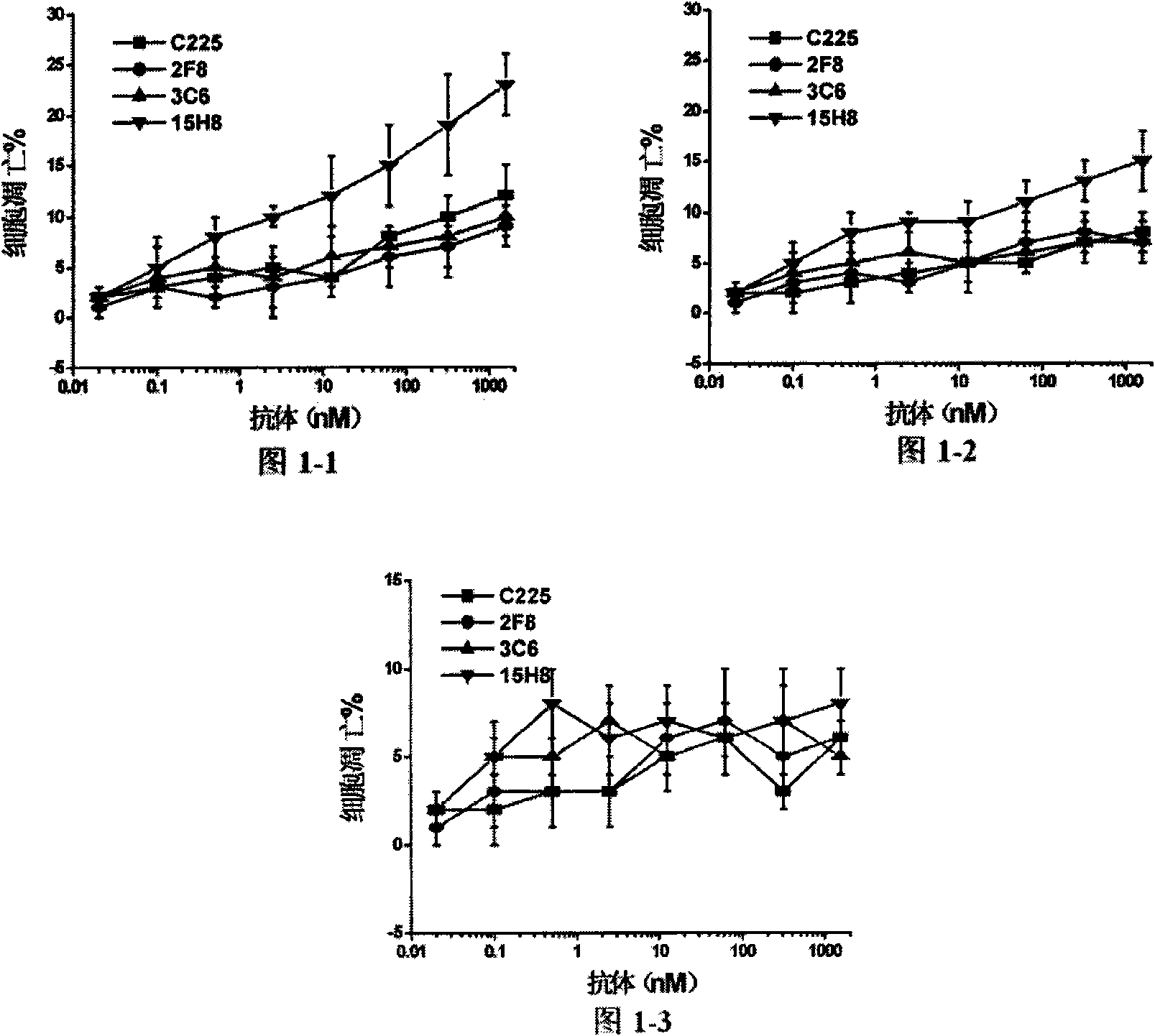
[0035] Experimental example 1. Apoptosis experiment of anti-EGFR antibody
[0036] Human breast cancer cells A431 (high expression of EGFR, ATCC number: CRL 1555), MDA-MB-231 (expression of EGFR, ATCC number: HTB-27) and MCF-7 (low expression of EGFR, ATCC number: HTB-22) ) were incubated with different dilutions of anti-EGFR antibodies (including 15H8, C225, 2F8, 3C6) at 37°C for 20 hours, and after washing the cells, use the AnnexinV / PI kit (BD company product) to detect the percentage of early apoptotic cells according to the product instructions . Anti-apoptosis assays such as figure 1 As shown: the ability of 15H8 antibody to kill A431 cells is significantly stronger than that of C225, 2F8 and 3C6 (when the antibody concentration is ≥0.5nM, P<0.05, t test, the same result is also obtained in MDA-MB-231 cells Confirmed (when the concentration of antibody concentration ≥ 0.5nM, P<0.05, t test). However, in MCF-7 cells with low expression of EGFR, the killing ability of 15...
experiment example 2
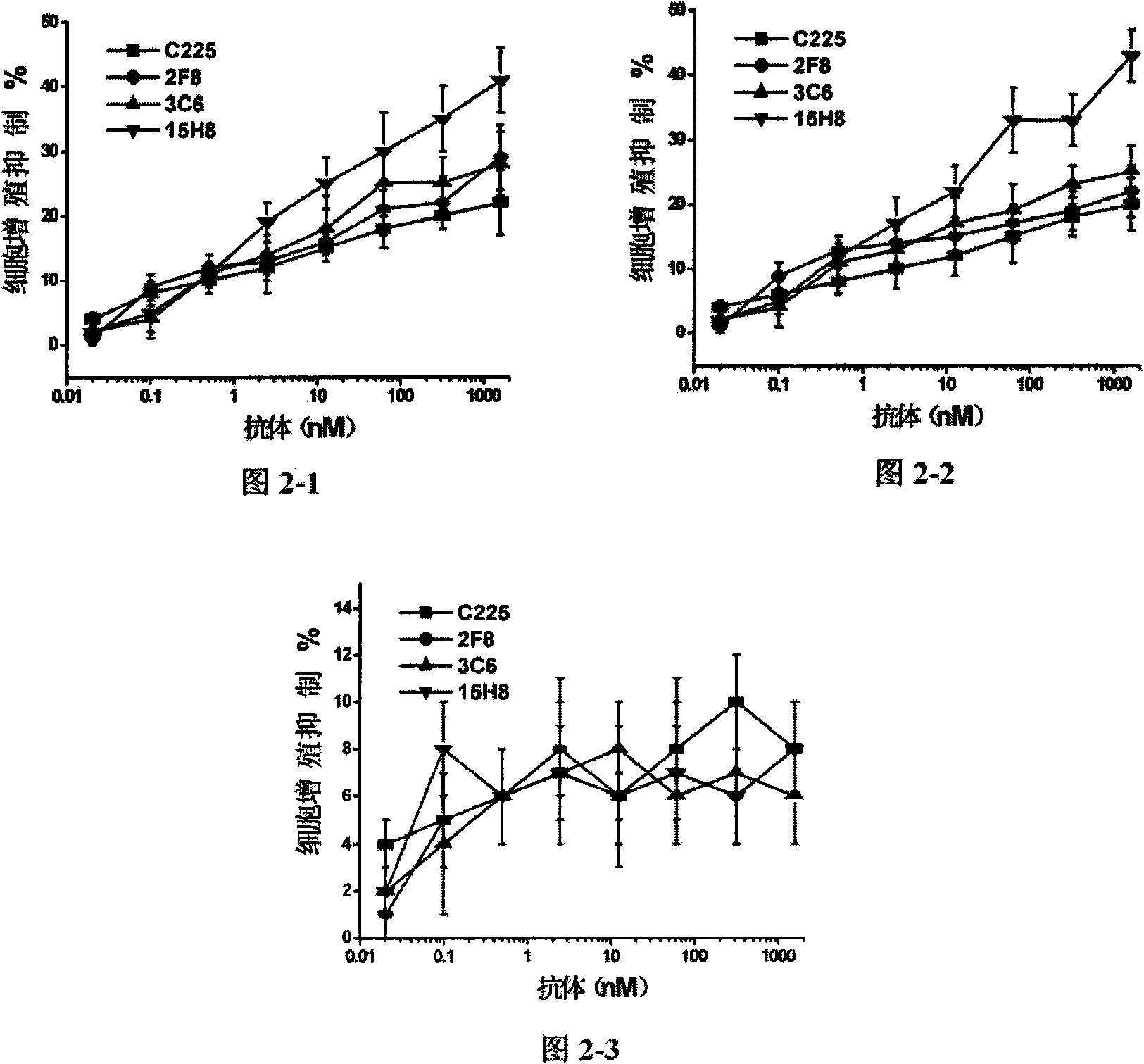
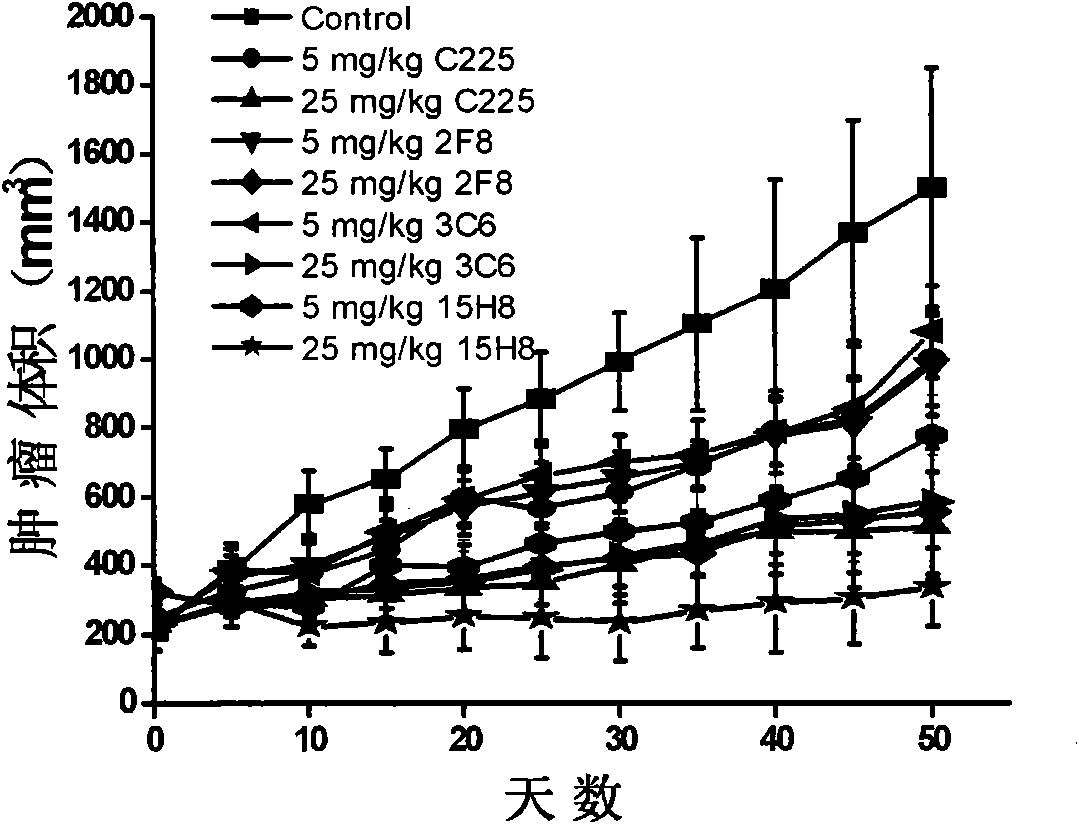
[0037] Experimental Example 2. Growth Inhibition Experiment of Anti-EGFR Antibody
[0038] Incubate human breast cancer cells A431, MDA-MB-231 and MCF-7 with different dilutions of anti-EGFR antibodies (including 15H8, C225, 2F8, 3C6) at 37°C, and stain with MTT staining on the fifth day Growth inhibition rate was calculated after reading. Growth inhibition assays such as figure 2 Shown: 15H8 antibody's ability to inhibit the growth of A431 cells was significantly stronger than that of C225, 2F8 and 3C6 (when the antibody concentration was ≥2.5nM, P<0.05, t test), the same result was also found in MDA-MB-231 Confirmed in cells (P<0.05, t-test at antibody concentration ≥ 2.5 nM concentration). However, in MCF-7 cells with low EGFR expression, the inhibitory ability of 15H8 antibody was similar to that of C225, 2F8 and 3C6. These results show that 15H8 antibody is EGFR specific for cell growth inhibition, and its ability to inhibit cells with high expression of EGFR is stron...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com