Method for preparing nevirapine
A technology of nevirapine and its compound, which is applied in the new field of preparation of nevirapine (Nevirapine), can solve problems such as environmental pollution, and achieve the effects of reducing safety hazards, low toxicity, and low price
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0037]
[0038] Synthesis of Compound IV:
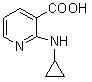
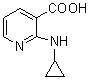
[0039] In the reaction flask, add 157.5 g (1.00 mol) of 2-chloronicotinic acid (compound II), 1000 ml of ethanol, 92.4 g (1.10 mol) of sodium bicarbonate and 500 ml of water, stir at room temperature for 30 minutes, and add 114 g of cyclopropylamine ( 2.0mol), heat up to 50°C and react for 24 hours. After the reaction, concentrate under reduced pressure to remove unreacted cyclopropylamine, ethanol and water to a volume of about 400ml, add 200ml of ethyl acetate for extraction, separate layers, and cool down the water layer To 0-5°C, add 4N hydrochloric acid dropwise to adjust PH=4-5, solid precipitates, stand for 2 hours, filter, filter cake is washed with ice water, and dried to obtain 152.8 g of yellow solid, yield 85.82% (based on compound II calculation).
[0040] 1HNMR (300MHz, CDCl3): δ (ppm) 0.35-0.52 (m, 4H), 1.32-1.36 (m, 1H),
[0041] 6.70-6.73 (m, 1H), 7.82-7.86 (m, 1H), 8.30-8.33 (m, 1H).
[0042] Synthesis of co...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com