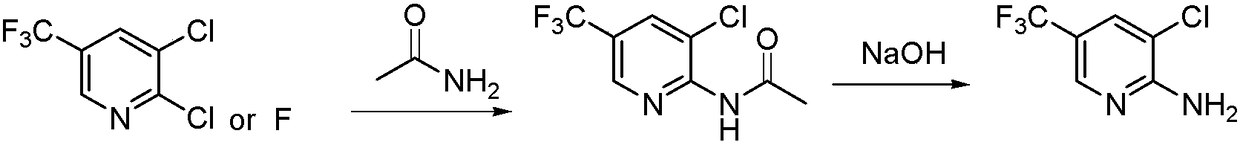
A method for preparing 2-amino-3-chloro-5-trifluoromethylpyridine
A technology of trifluoromethylpyridine and amino group, applied in the field of chemical raw material preparation, can solve the problems of high reaction temperature, high risk, unsuitability for use, etc., and achieves the effects of simple operation, low environmental pollution and good product quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Add 51.5g (0.92mol) potassium hydroxide and 330ml (4mol) dimethyl sulfoxide to the reaction flask, stir and cool down to 20°C, slowly add 42.7g (0.7mol) acetamide, then dropwise add 100g (0.46mol) ) 2,3-dichloro-5-trifluoromethylpyridine (the molar ratio is: 2,3-dichloro-5-trifluoromethylpyridine: potassium hydroxide: acetamide: dimethyl sulfoxide=1: 2:1.5:10), after the dropwise addition, keep the reaction at this temperature. After the raw materials have reacted completely, add 500 ml of water to the reaction solution, adjust the pH to 1-2 with hydrochloric acid, and gradually precipitate solids. After stirring for 1 hour, Filtration, washing with water, and vacuum drying gave the product 3-chloro-2-acetylamino-5-trifluoromethylpyridine as a yellow powder, 108.5 g, with a yield of 98.63%.
[0023] 108.5g (0.45mol) 3-chloro-2-acetylamino-5-trifluoromethylpyridine, 273ml (6.8mol) methanol, 36g (0.9mol) sodium hydroxide (molar ratio: 3-chloro-2- Acetylamino-5-trifluorom...
Embodiment 2
[0025] Add 50.4g (0.9mol) potassium hydroxide and 360ml (4.1mol) N,N-dimethylacetamide to the reaction flask, stir and cool down to 35°C, slowly add 36.5g (0.6mol) acetamide, then drop Add 100g (0.46mol) 2,3-dichloro-5-trifluoromethylpyridine (the molar ratio is: 2,3-dichloro-5-trifluoromethylpyridine: potassium hydroxide: acetamide: N,N -Dimethylacetamide=1:2:1.3:9), keep the reaction at this temperature after the dropwise addition is completed, after the raw materials have reacted completely, add 500 ml of water to the reaction solution, adjust the pH to 1-2 with hydrochloric acid, Solids were gradually precipitated, and after stirring for 1 hour, filtered, washed with water, and dried in vacuo to obtain the product 3-chloro-2-acetylamino-5-trifluoromethylpyridine, a yellow powder, 103 g, with a yield of 93.71%.
[0026] 103g (0.43mol) 3-chloro-2-acetylamino-5-trifluoromethylpyridine, 300ml (6.45mol) ethanol, 51.6g (1.29mol) sodium hydroxide (molar ratio: 3-chloro-2- Acetyl...
Embodiment 3
[0028] Add 55.2g (1.4mol) sodium hydroxide and 360ml (4.1mol) N,N-dimethylformamide to the reaction flask, stir and cool down to 40°C, slowly add 47.4g (0.8mol) acetamide, then drop Add 100g (0.46mol) 2,3-dichloro-5-trifluoromethylpyridine (the molar ratio is: 2,3-dichloro-5-trifluoromethylpyridine: sodium hydroxide: acetamide: N,N -Dimethylformamide=1: 3: 1.7: 12), keep the reaction at this temperature after the dropwise addition is completed, after the raw materials have reacted completely, add 500 milliliters of water to the reaction solution, adjust the pH to 1-2 with hydrochloric acid, Solids were gradually precipitated, and after stirring for 1 hour, filtered, washed with water, and dried in vacuo to obtain the product 3-chloro-2-acetylamino-5-trifluoromethylpyridine, a yellow powder, 106 g, with a yield of 96.49%.
[0029] With 106g (0.44mol) 3-chloro-2-acetylamino-5-trifluoromethylpyridine, 264ml (4.4mol) ethanol, 49.3g (0.88mol) potassium hydroxide (molar ratio is: 3-...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com